Continuing Education Activity
Although more than 90 percent of identified thyroid nodules are clinically insignificant benign lesions, in 4 to 6.5 percent of cases, these nodules are due to thyroid cancer. This activity reviews the evaluation and management of thyroid nodules and highlights the role of interprofessional team members in collaborating to provide well-coordinated care and enhance patient outcomes.
Objectives:
- Describe the epidemiology of benign and malignant thyroid nodules.
- Describe the evaluation of thyroid nodules.
- Outline the management strategies for different types of thyroid nodules.
- Review the importance of enhancing care coordination among the interprofessional team to ensure proper evaluation and management of thyroid nodules.
Introduction
The American Thyroid Association (ATA) defines the thyroid nodule as a discrete lesion within the thyroid gland. It is radiologically distinct from the surrounding thyroid parenchyma.[1] Nodules may be solitary, multiple, cystic, or solid.[2]
Nodules in the thyroid gland are a common entity and are detected in approximately 5% to 7% of the adult population by physical examination alone. However, autopsy data have shown a 50% prevalence of thyroid nodules larger than one centimeter in patients without previously diagnosed thyroid disease.[3][4] Nodules are found with increasing frequency, likely due to the widespread use of modern imaging modalities, particularly ultrasound (US), but also computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET).[2]
Although more than 90% of detected nodules are clinically insignificant benign lesions,[4] thyroid nodules are clinically important as they may represent thyroid cancer in approximately 4.0% to 6.5% of cases.[5]
Etiology
A broad spectrum of disorders is associated with nodules of the thyroid, from benign to malignant conditions that may have indolent or very aggressive clinical courses.[5] Approximately 23% of solitary nodules represent a dominant nodule within a multinodular goiter.[6]
Ionizing radiation is a known risk factor for both benign and malignant nodules of the thyroid. This population may develop thyroid nodules at a rate of 2% annually.[6] The incidence of malignancy has been documented as high as 20% to 50% in palpable nodules of previously irradiated thyroids glands.[7]
Other factors that lead to an increased risk of thyroid nodules and goiter include smoking, obesity, metabolic syndrome, alcohol consumption, increased levels of insulin-like growth factor-1, and uterine fibroids. Factors associated with possible decreased risk may include the use of oral contraceptives and statins.[7]
Thyroid nodules may be classified as neoplastic and non-neoplastic. Neoplastic nodules may be benign or malignant, with benign neoplastic including non-functioning and functioning nodules. Non-neoplastic nodules include hyperplastic and inflammatory nodules.[2]
Colloid nodules represent adenomatous benign neoplasms, are the most common thyroid nodules, and do not pose an increased risk of malignancy. While most follicular adenomas are benign, they share characteristics with follicular carcinomas.[6]
Thyroid carcinomas may be classified as non-medullary thyroid cancers (NMTCs), arising from epithelial cells and constituting approximately 95% of all thyroid malignancies, or medullary thyroid cancer (MTC) arising from the calcitonin-producing parafollicular cells of the thyroid. Twenty percent of MTCs are familiar and may occur as part of multiple endocrine neoplasia (MEN) syndromes.[7]
Epidemiology
Prevalence depends on the method of screening and the population evaluated. The risk of thyroid nodules is higher with increasing age, female gender, iron deficiency, and history of thyroid radiation.[8] Long-term survivors of hematopoietic stem cell transplantation are at higher risk for secondary thyroid carcinoma with a relative risk of 3.26.[9]
In the adult population, physical examination alone may show a prevalence of 5% to 7% of thyroid nodules. Ultrasound shows a prevalence of 20% to 76% in this same population, which correlates to autopsy findings.[3][4][5]
Thyroid nodules are approximately 4 times more common in women than men and occur more often in individuals living in iodine-deficient geographic areas.[6] A 20-year surveillance study estimated a prevalence of 0.8% and 5.3% in men and women, respectively.[10] However, the rate of cancer is twice as high in men as in women (8% versus 4%).[11]
Pathophysiology
The pathophysiology of a thyroid nodule will vary depending on the lesion. Several disorders may cause thyroid nodules. The most common type is benign macrofollicular nodules, representing either monoclonal adenomas or colloid nodules in multinodular goiter. The latter represents the expansion of relatively monoclonal cells replicating in a nodular fashion.[12]
Follicular neoplasms may represent a diagnostic problem as these only differ from follicular carcinomas by lack of vascular or capsular invasion.[4][13]
The association between thyroid irradiation and tumorigenesis is well-known. Radiation may cause a wide range of somatic mutations that increase the risk of cancer, particularly in radiation-sensitive organs such as the thyroid. Compared to adults, children have a higher risk of thyroid cancer after irradiation; this is most likely due to the higher proliferative activity of the thyroid tissue in younger individuals.[14]
RET proto-oncogene translocations have been found in thyroid malignancies associated with ionizing irradiation. The presence of RET/PCT translocations has been described in follicular adenomas presenting after irradiation.[15]
Histopathology
The 2 most commonly used diagnostic techniques for assessing cytopathology of thyroid nodules are FNA and fine-needle capillary sampling (FNC). The National Cancer Institute recommends using the Bethesda classification that stratifies cytologic findings into 6 major categories, each showing different subsequent evaluation and management.[16][17]
The Bethesda system diagnostic categories for reporting thyroid cytopathology describes[1][16][18]:
- Non-diagnostic: Represents an inadequate sample with an insufficient number of follicular cells
- Benign, normal thyroid tissue, showing nodules of adenomatous or multinodular goiters, common entities include adenomatous nodules, Hashimoto thyroiditis, and subacute granulomatous thyroiditis.
- Follicular lesion of undetermined significance (FLUS) or atypia of undetermined significance (AUS): proposed for lesions that are not convincingly benign. AUS shows lesions with nuclear atypia and lesions with extensive oncocytic changes, although not enough to be classified as a Hürthle cell neoplasm. FLUS show a combined microfollicular and macrofollicular pattern.
- Follicular neoplasm or suspicion for follicular neoplasm includes microfollicular or cellular adenomas. Since FNA only samples a portion of the nodule, surgical excision is required to determine if a microfollicular lesion is benign or malignant; microfollicles are identified, the colloid is absent or scant, and cells are more crowded than in macrofollicular nodules.[19]
- Suspicious for malignancy: Includes lesions with features of malignancy that are not definite for thyroid cancer
- Malignancy: Cytology will differ on the different types of possible thyroid malignancies. For papillary cancers, microscopy shows large cells with ground-glass cytoplasm, prominent nucleoli, and intranuclear cytoplasmic inclusions. Medullary cancer shows disperse cells showing eccentrically displaced nuclei and slightly granular cytoplasm usually configured as a teardrop. Anaplastic cancer shows marked pleomorphism, bizarre giant cells, and spindle cells.
History and Physical
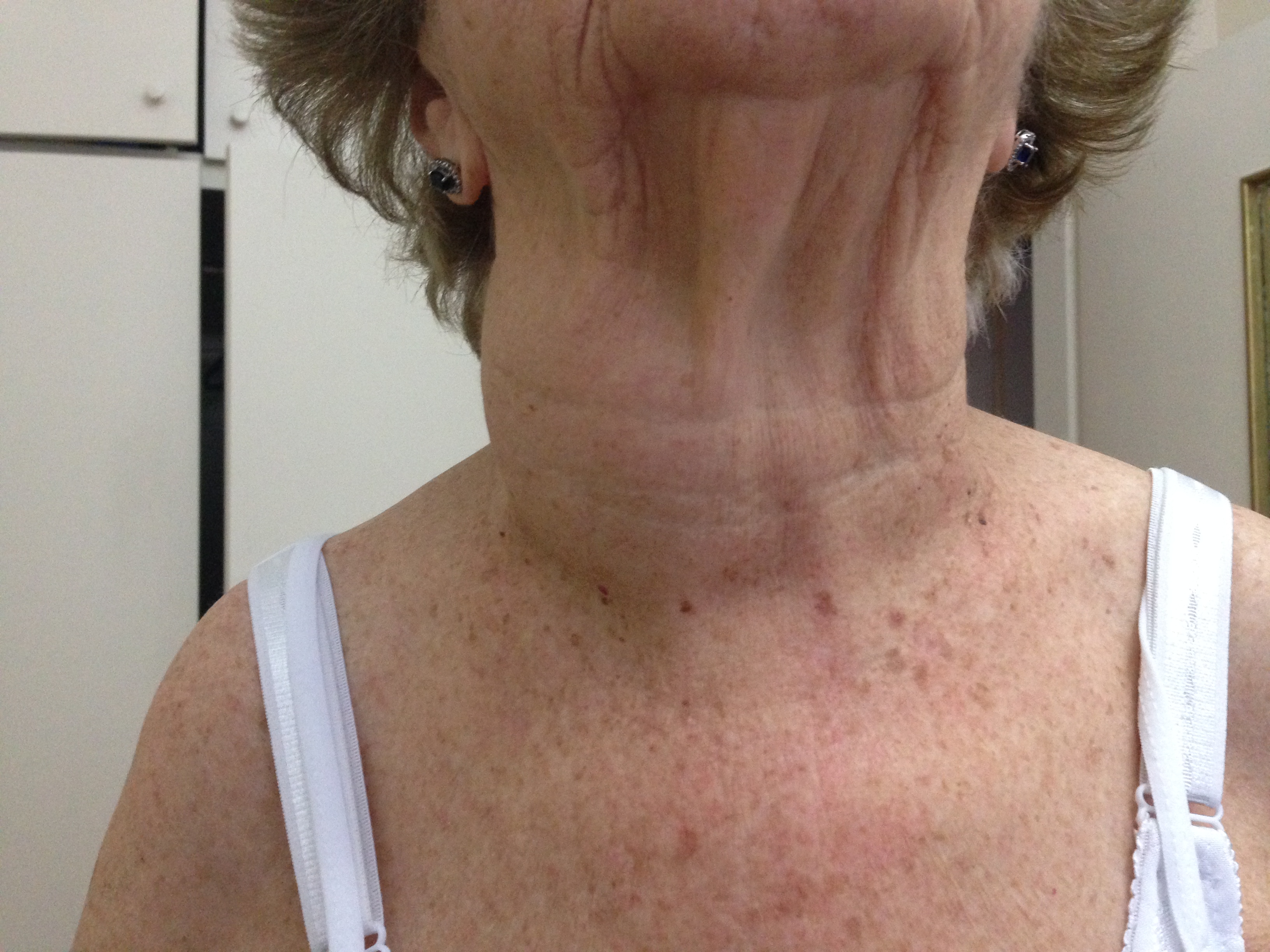
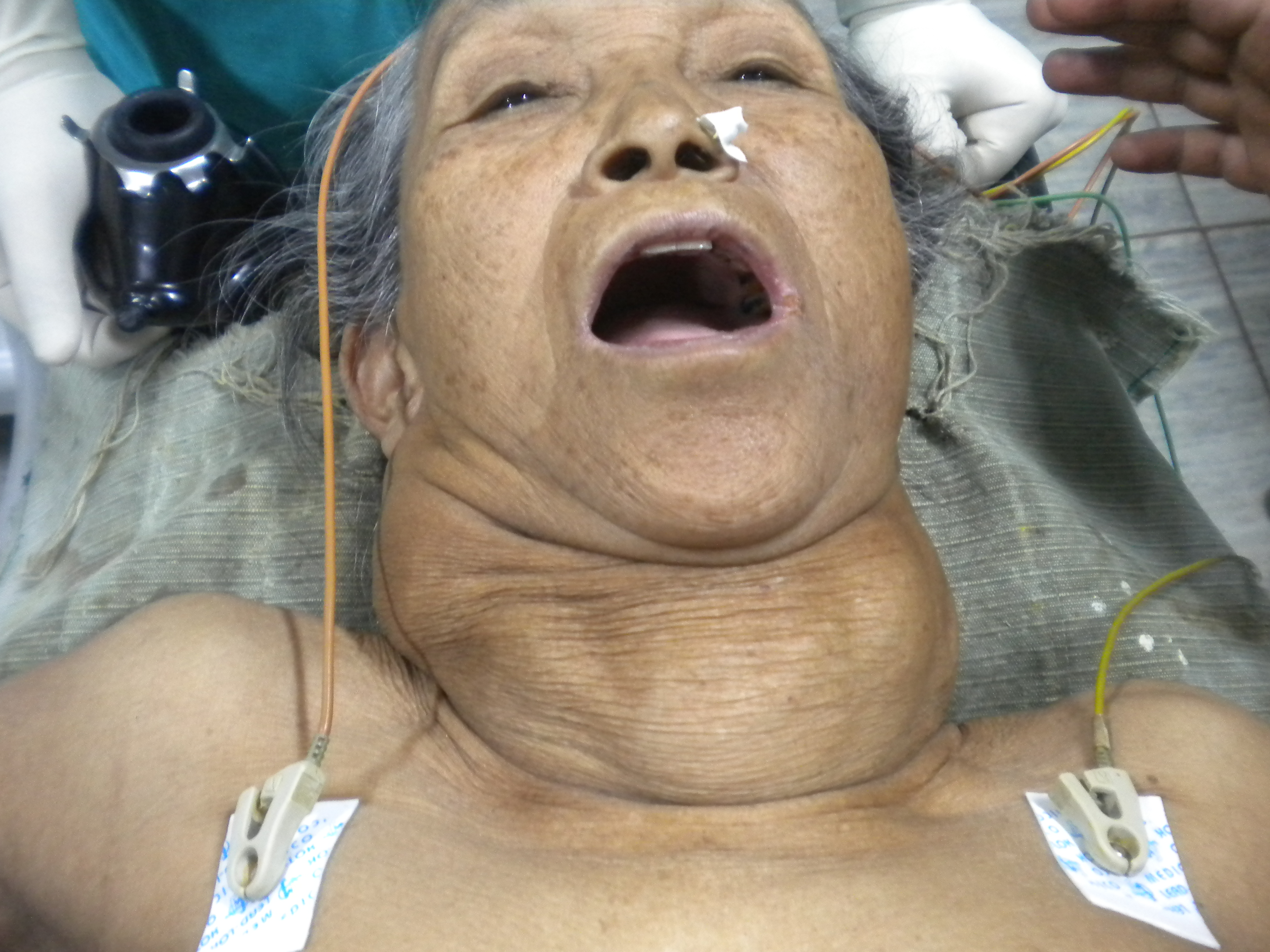
Most patients will present with a large palpable nodule in the anterior neck, or an incidental nodule is found on imaging studies performed for other reasons. Most thyroid nodules are asymptomatic, and most individuals with thyroid nodules are euthyroid, with less than 1% of nodules causing thyroid disease. Some patients may complain of neck pressure or pain, particularly when spontaneous hemorrhage occurs.[6]
Palpation of the thyroid is the easiest but least sensitive method for detecting thyroid nodules, with a prevalence of 4% to 7%.[8] Physical examination findings that should raise concern for malignancy include nodules larger than 4 cm in size (approximately 19% risk for malignancy), firmness on palpation, fixation to adjacent tissues, cervical lymphadenopathy, and vocal fold paralysis. In some patients, the physical examination may be limited by body habitus.[3]
The combined physical examination findings of a solitary nodule along in addition to cervical lymphadenopathy (greater than 1 cm) and vocal fold paralysis, have an approximate positive predictive value of 100% for thyroid malignancy.[3]
Appropriate social history is important and may aid in identifying patients with MEN II syndrome. These are at higher risk for pheochromocytomas and need appropriate workup before surgical intervention.[7]
Evaluation
Initial evaluation for patients with a thyroid nodule should include history, physical examination, measurement of thyroid stimulating hormone (TSH), and thyroid ultrasound to characterize the nodule. Measurement of TSH by itself may be able to detect subtle thyroid dysfunction.[20]
Diagnostic workup includes serum markers, cytology by fine-needle aspiration (FNA), genetic markers, immunohistochemical markers, and several imaging modalities, most commonly ultrasonography, but also elastography, MRI, CT, and 18FDG-PET scans.[3][5][7]
In every patient with a thyroid nodule, thyroid-stimulating hormone (TSH) measurement should be the initial test and be used as a guide for further management. A normal or high TSH commonly raises concerns as the risk of malignancy increases in parallel with the level of serum TSH. A low TSH, however, usually favors a benign nodule. The next step in a patient with low TSH is to evaluate the possibility of an autonomously functioning thyroid nodule using iodine-123 (123-I) or pertechnetate scintigraphy scan. Autonomously functioning thyroid nodules are most commonly benign and rarely require further diagnostic workup.[5][7]
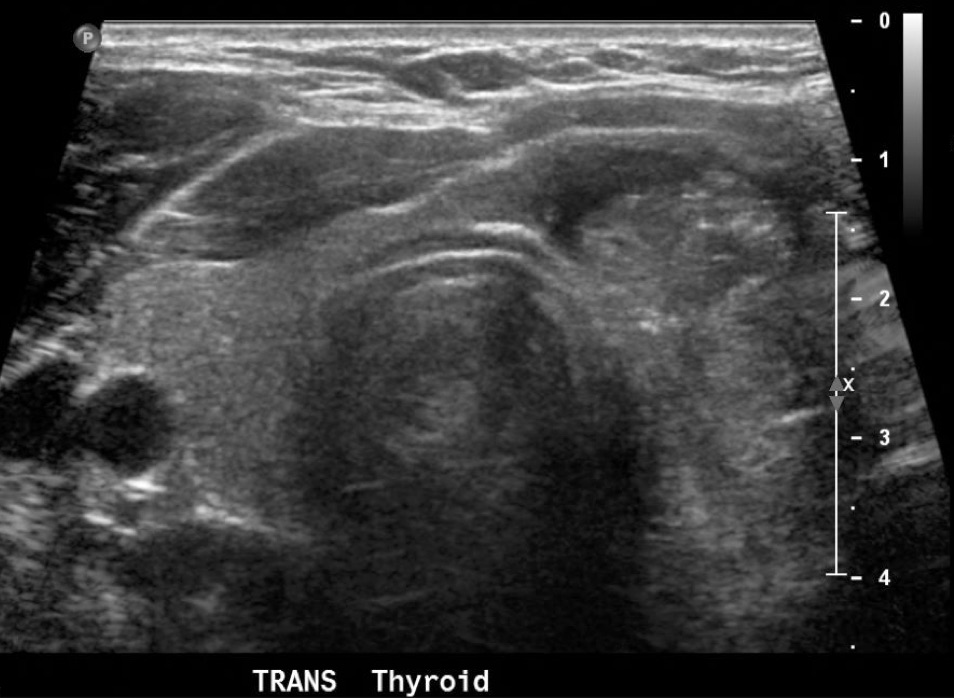
Ultrasound (US) of the thyroid is an important imaging modality used to evaluate thyroid nodules. It provides information regarding dimensions, structure, and parenchymal changes and may detect lesions as small as 2 mm. It is commonly used to differentiate between benign and malignant lesions to avoid the unnecessary use of invasive procedures. Several features have been associated with malignancy and found to be independent risk factors. These include microcalcifications, irregular margins, hypoechogenicity, taller-than-wide shape, and increased vascularity.[3][5][7]
US has a high sensitivity for the detection of thyroid nodules that are too small to palpate. However, these nodules may have an indeterminate clinical significance.[8] A malignancy rate of 24% was found for incidental thyroid nodules in patients previously diagnosed with a primary non-thyroid malignancy. This substantially exceeds the 5% rate of expected malignancy in patients without another known primary tumor.[9]
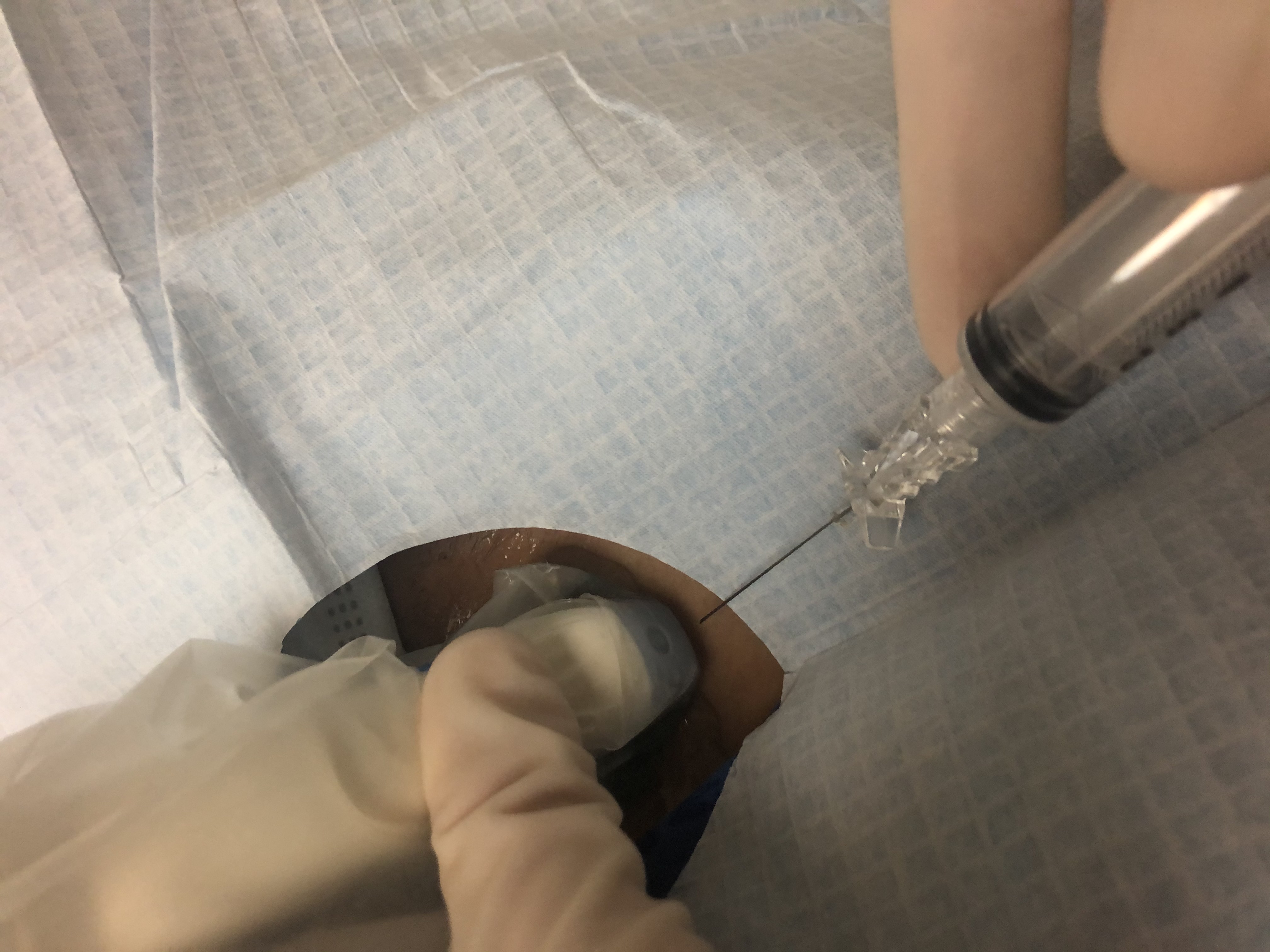
In conjunction with the US, FNA forms the cornerstone for thyroid nodule evaluation, representing the most cost-effective diagnostic tool used in the assessment of nodules of the thyroid. Its use under US guidance is preferred over palpation-guided biopsies associated with higher rates of false-negative results and non-diagnostic cytology.[5]
The decision to perform FNA should be based on individual risk-stratification using the patient’s history, clinical and US findings. Nodules smaller than one centimeter are biopsied when there is more than one suspicious US characteristic, cervical lymphadenopathy, or high-risk history. Otherwise, a cutoff size of 1 centimeter is used for solid nodules with only 1 suspicious US feature.[5]
It is suggested to perform a US-FNA in patients with[2]:
- Nonpalpable thyroid nodules larger than 1 cm
- Palpable nodules smaller than 1.5 cm
- Deeply found nodules
- Nodules in proximity to blood vessels
- Nodules after nondiagnostic conventional FNA cytology
- Cystic or mixed nodules, especially if a previous conventional FNA was nondiagnostic
- Coexistence of nonpalpable lymphadenopathy
Cystic or spongiform lesions are considered to pose a low risk for malignancy and are either monitored or biopsied if larger than 2 centimeters.[5]
Treatment / Management
Initial management of thyroid nodules depends on the type of lesion found, US characteristics, and whether it is functional or not. FNA results will ultimately guide treatment.[2]
FNA cytology results provide 6 major diagnostic categories (Bethesda classification), all of which indicate different subsequent management.[16]
- Non-diagnostic: Cancer risk 5% to 10%
- Benign: Cancer risk 0% to 3%
- Atypia of undetermined significance or follicular lesion of undetermined significance: Cancer risk 10 to 30%
- Follicular neoplasm (or suspicious for follicular neoplasm): Cancer risk 25% to 40%
- Suspicious for malignancy: Cancer risk 50% to 75%
- Malignant: Cancer risk 97% to 99%
Non-diagnostic biopsies (Bethesda I) are considered cytologically inadequate. The absence of malignant cells should not be interpreted as a negative biopsy if scant follicular tissue is obtained. FNA is usually repeated in 4 to 6 weeks.[21]
Patients with benign nodules (Bethesda II), such as macrofollicular, colloid adenomas, nodular goiter, and Hashimoto thyroiditis, are usually followed without surgery. Periodic US monitoring initially at 12 to 24 months, with increasing intervals, is preferred. If the US shows highly suspicious findings, then FNA should be repeated within 12 months despite a benign initial biopsy.[1][4]
For nodules with indeterminate cytology (Bethesda III and IV), the approach varies with institutional practices. Some institutions obtain an additional FNA sample for molecular testing, while other centers repeat the FNA after 6 to 12 weeks. A radionuclide scan may also be obtained if repeat aspirates show only architectural atypia.[17][22]
For nodules that fall within the category of Bethesda V, suspicious for malignancy, treatment should include surgery. Molecular markers should not be used. Bethesda VI includes papillary cancer, MTC, thyroid lymphoma, anaplastic cancer, and cancer metastatic to the thyroid. These patients should also be referred for surgery.[3]
Differential Diagnosis
While most nodules and masses presenting in the anterior neck represent benign thyroid nodules or cysts, malignancy should still be excluded, particularly in patients at risk for thyroid cancer.[23]
Congenital neck masses may present in the anterior neck. These are usually present at birth, but some may present in adulthood. Older age of presentation should raise concern for possible malignancy. Carcinomas of the tongue, tonsil, and thyroid cancer may present as cystic neck masses.[23][24]
Inflammatory neck masses usually represent enlarged lymph nodes which may be viral or bacterial in etiology. These are commonly located superficial, deep, or posterior to the sternocleidomastoid muscle, and anterior to the trapezius muscle.[23]
Some non-thyroid neoplastic disorders may present as metastatic neck masses; these are most commonly associated with squamous cell carcinoma from the aerodigestive tract.[25]
Prognosis
Most thyroid nodules are benign. Concern for malignancy is increased by initial findings such as normal to high serum TSH level, history of irradiation or MEN, and some ultrasonographic features, including microcalcifications and irregular margins hypoechogenicity, taller-than-wide shape, and vascularity. [5] While solitary nodules pose a higher risk for malignancy than nodules within a multinodular thyroid, the overall risk of malignancy will be approximately equal due to the additive risk of each nodule in a patient with a multinodular gland.[3]
The prognosis for thyroid malignancy will greatly vary depending on the histological type and subtype of cancer in addition to several individual characteristics, including age at diagnosis, size of the primary tumor, presence of soft tissue invasion, or distant metastasis.[26][27]
Most patients with papillary thyroid cancer do not die of the disease. One case series on patients with non-metastatic papillary thyroid cancer showed malignancy-related mortality of 6%.[13]
Other factors associated with an increase of malignancy recurrence or death include male gender, mediastinal lymph node involvement, delay in primary surgical therapy of more than 1 year after detection of a nodule, and multicentricity of the intrathyroidal tumor.[28]
Follicular cancer generally occurs in older patients and follows an aggressive course. It is commonly associated with distant metastasis and higher mortality than papillary thyroid cancer.[29]
Complications
Hyperthyroidism symptoms may be a complication in patients with functional thyroid nodules. Clinical manifestations will include those of hyperthyroidism, such as sweating, palpitations, and impaired glucose tolerance. However, the majority of thyroid nodules are benign [4], and most patients will have no symptoms.[30]
A minority of patients, particularly those with cystic thyroid lesions, may present with thyroid pain, which may indicate sudden hemorrhage or hemorrhagic infarction.[30]
Deterrence and Patient Education
Patients should be reassured that up to half of the population have at least one thyroid nodule and that benign conditions cause most of these.[3] Many nodules are incidental findings during radiological procedures or routine physical examinations, and the significance of such findings should be determined based on the individual risk factors for each patient.[1][4][5]
Enhancing Healthcare Team Outcomes
Incidentalomas are detected with increasing frequency due to the widespread use of imaging studies performed for different reasons, the risk of unnecessary diagnostic intervention and treatment increases. This poses the challenge of determining the clinical significance of these findings.[1][8]
Some palpable nodules will not be radiologically distinct from the surrounding thyroid parenchyma and will therefore not meet the strict definition for thyroid nodules.[1] In these cases, follow-up should be based on individual risk factors.
US screening for non-palpable nodules in high-risk population groups, such as those previously exposed to radiation, is still controversial due to a lack of compelling evidence supporting this practice. Health-care providers should consider that screening for malignancy is usually supported by[1]:
- A clear demonstration that the patient is indeed at risk for such malignancy
- Demonstration that screening will allow for the detection of the disease at an earlier stage
- Early diagnosis will have an impact on the subsequent outcome
There is no current evidence supporting US screening for nonpalpable thyroid nodules in individuals previously exposed to radiation.[31] As such, surveillance may be guided by the individual assessment of each patient’s risk factors, including the dose of radiation, the age of exposure, and potential harms and benefits of US surveillance.