Continuing Education Activity
Most cerebral aneurysms are asymptomatic and small; most are incidentally found during brain imaging or an autopsy. A ruptured aneurysm is a serious and life-threatening emergency that requires prompt treatment. The symptoms and signs of cerebral aneurysms are distinct if the appropriate history and imaging modalities guide clinicians. In particular, neurological consultation best determines if hospitalization or critical care is needed with further monitoring.
Clinicians participating in this course review the broad differential diagnosis of an aneurysm and the alarming signs that indicate an emergency. The differential diagnosis leads to a high suspicion of index for saccular aneurysms, which informs the best management plan. Furthermore, several modifiable and non-modifiable risk factors may prevent additional aneurysms or prevent the first aneurysm from occurring. This activity reviews the evaluation and treatment of saccular aneurysms (cerebral and aortic) and highlights the role of the interprofessional team in the care of patients at risk for cerebral aneurysms.
Objectives:
Identify the etiology of saccular aneurysms by utilizing patient history to guide a differential diagnosis.
Determine the appropriate evaluation of saccular aneurysms, incorporating imaging modalities.
Compare the management options available for saccular aneurysms.
Describe interprofessional team strategies for improving care coordination and communication in patients with saccular aneurysms.
Introduction
An aneurysm is an abnormal dilatation or bulging in a blood vessel due to the intrinsic weakness of the vessel wall.[1] Aneurysms can affect any blood vessel, but they are most commonly seen in arteries rather than veins. An aneurysm can be a true aneurysm or a false aneurysm. A true aneurysm has all 3 layers of the arterial wall (intima, media, and adventitia). A false aneurysm, also known as a pseudoaneurysm, involves only the artery's outer layer (adventitia).
Depending on their shape, aneurysms can be saccular or fusiform. Cerebral aneurysms are 90% saccular aneurysms (also known as berry aneurysms), unlike aortic aneurysms, which are about 94% fusiform. Aneurysms can be classified based on their location in the body. Depending on the etiology, they can be dissecting or mycotic aneurysms.
This review focuses on saccular cerebral and aortic aneurysms. Saccular cerebral aneurysms can also be classified by size:
- Small: 5 mm or less
- Medium: 6 mm to 14 mm
- Large: 15 mm to 25 mm
- Giant: greater than 25 mm [2]
Most saccular aortic aneurysms are located in the descending thoracic aorta.[3] Most cerebral aneurysms are asymptomatic and small, and they are incidentally found during brain imaging or an autopsy.[4] About 85% of cerebral aneurysms are located in the anterior circulation at the arterial bifurcations on the circle of Willis and the middle cerebral artery bifurcation.[4][5]
Etiology
Although the etiology of cerebral saccular aneurysms is not completely understood, they are thought to be associated with hemodynamically induced degenerative vascular changes. Inflammatory changes also play a role in their formation. Most saccular aneurysms are acquired, but they can be inherited.
Acquired risk factors of saccular aneurysm formation may include the following:
- Advanced age
- Hypertension
- Excessive alcohol consumption
- Cigarette smoking
- Atherosclerosis of the cerebral arteries
- Trauma to the head
- Use of illicit drugs such as cocaine
- Estrogen deficiency [4][6]
Inherited risk factors of saccular aneurysm formation may include the following:
- Polycystic kidney disease
- Arteriovenous malformation
- Ehlers-Danlos syndrome
- Marfan syndrome
- Loeys-Dietz syndrome
- Fibromuscular dysplasia
- Hereditary hemorrhagic telangiectasia
- Tuberous sclerosis
- Family history of aneurysms
- Alpha-glucosidase deficiency
- Alpha 1-antitrypsin deficiency
- Coarctation of the aorta
- Klinefelter syndrome
- Noonan syndrome
- Female gender [4][6]
Family history is the strongest indicator of rupture among non-modifiable risk factors. Compared to the general population, first-degree relatives of persons with cerebral aneurysms or previous subarachnoid hemorrhage have a 3 to 7 times higher risk.[7] Nicotine exposure promotes aneurysmal rupture through actions on vascular smooth muscle cells.[8]
The etiology of saccular aortic aneurysms is almost similar to saccular cerebral aneurysms. The most common risk factor of saccular aortic aneurysms is atherosclerosis; other less common risk factors include aortic infections, trauma, chronic inflammatory or autoimmune conditions (eg, Behçet disease, giant cell arteritis, rheumatoid arthritis, Takayasu arteritis, systemic lupus erythematosus, ankylosing spondylitis), presence of bicuspid aortic valve, or previous aortic surgeries.[3] In addition to the above genetic conditions listed under cerebral aneurysms, saccular aortic aneurysms are associated with Turner syndrome.
Epidemiology
The prevalence of cerebral aneurysms is estimated to be between 3.2% to 4% in the general population without inherited risk factors. The most common age of presentation is between 30 and 60 years (mean age of 50 years with a 1:1 gender ratio).[9] After 50 years, the prevalence increases in women compared to men and may approach a 2:1 ratio.[9] Approximately 20% to 30% of patients with cerebral aneurysms have multiple aneurysms.[10] The incidence of cerebral aneurysm rupture causing SAH is estimated to be 6 to 16 per 100,000 individuals, and this accounts for 30,000 cases per year in the United States alone.[11]
The risk of rupture of an unruptured cerebral aneurysm is 1% to 2% per year.[7] Approximately 10% to 30% of patients with aneurysm rupture die before reaching the hospital, and only 30% have a positive outcome after appropriate treatment.[4] Cerebral aneurysm rupture accounts for 0.4% to 0.6% of all deaths.[4] Ruptured aneurysms have a 20% to 50% risk of rebleeding in the first 2 weeks if untreated. Cerebral aneurysms are uncommon in the pediatric population. Approximately 85% of the cerebral aneurysms are located in the anterior circulation on the circle of Willis and the middle cerebral artery bifurcation. The most common sites of cerebral aneurysms include:
- Junction of the anterior cerebral artery with the anterior communicating artery
- Junction of the internal carotid artery with the posterior communicating artery
- Bifurcation of the middle cerebral artery
In the posterior circulation, the most common sites often include the distal basilar bifurcation (basilar tip), the junction of the basilar artery and the superior or anterior inferior cerebellar arteries, and the junction of the vertebral artery with the posterior inferior cerebellar artery.[12]
Results from a study of 284 patients with 322 saccular aortic aneurysms showed that 68% were found in the thoracic aorta, 24.2% in the abdominal aorta, 7.1% in the arch of the aorta, and 0.6% in the ascending aorta.[3] When aortic aneurysms are studied, aortic saccular aneurysms comprise 6%; fusiform aneurysms comprise 94%.[13] As saccular aortic aneurysms are less common than fusiform aneurysms, most epidemiological studies report data that includes any aortic aneurysms. The true incidence and prevalence of saccular aortic aneurysms are unknown.
Pathophysiology
The pathophysiology for the formation and growth of cerebral and aortic saccular aneurysms is a multifactorial process that leads to degenerative changes in the blood vessel wall.[4][6][7] These degenerative changes could be due to collagen deficiency or congenital weakness of the arterial wall layers. Alteration of the internal elastic lamina develops from hemodynamic shear stress, hypertension, turbulent blood flow, and atherosclerotic deposits, which are associated with focal defects, disorganization, or absence of the media layer.[7][14][15][16]
Chronic inflammation and immunologic response are recognized as important factors in developing atherosclerosis. Inflammatory mediators such as T-cells and macrophages may be associated with histologic changes in the arterial wall, leading to cerebral aneurysms' formation, progression, and rupture.[5][7][15][17] Endothelial dysfunction is considered the first step in the formation of cerebral aneurysms.[16]
Three Theories on the Formation of Berry Aneurysms
- The congenital theory: Aneurysms are caused by improper development or an innate weakness of the artery wall.
- The degeneration theory: Aneurysms are brought on by inherited degenerative changes in the vessel wall.
- A combination of degenerative and developmental factors is to blame for the aneurysms.
Changes that Facilitate Berry Aneurysm Formation
- Vessel wall changes: Vascular remodeling causes vessel wall changes. Type I collagen increases, and fibronectin is dispersed in the wall. Damage to the vascular endothelium due to hemodynamic stress is the inciting event for aneurysm formation. The damage leads to an inflammatory cascade that causes the progression and rupture of intracranial aneurysms. The earliest change in aneurysm formation is a partial loss of endothelium; the latest is intimal swelling.
- Inflammatory changes: These are caused by endothelial dysfunction, and the main inflammatory mediators are NF-κB, Ets-1, MCP1, IL-1β, and nitric oxide.
- Genetic changes: More than 500 differentially expressed genes are identified from intracranial aneurysm tissue.[7]
Histopathology
The Structure of the Wall of Intracranial Arteries:
- The intima: consists of a single layer of endothelial cells (ECs)
- The media: constituted by circumferentially oriented smooth muscle cells (SMCs) within a dense network of types I and III collagen, elastin fibers, and proteoglycans
- The adventitia: the outer connective tissue layer made of collagen fibers and fibroblasts
- Subendothelial layer: connective tissue layer containing several SMCs beneath the EC layer
The Internal Elastic Lamina:
- Separates the intima and media
- Made of elastic fibers that are primarily concentrated in this layer
- The critical structure involved in the formation of intracranial aneurysms at the structural level
- The disintegration of this layer is a major factor in the pathogenesis of aneurysm formation (see Table. The Differences Between Intracranial and Extracranial Arteries)
Table. The Differences Between Intracranial and Extracranial Arteries
| Feature |
Intracranial Artery |
Extracranial Artery |
| Muscle Content |
Less-developed media with a lesser number of smooth muscles |
Muscular media with abundant elastic fibers |
| Internal Elastic Lamina |
Well-developed with elastic fibers |
Less prominent |
| External Elastic Lamina |
Absent |
Present |
| Adventitia |
Thin |
Thick with abundant elastic fibers [18] |
History and Physical
Cerebral Aneurysms
The majority of unruptured cerebral aneurysms are asymptomatic (85% to 90%), and they are usually found during brain imaging for other reasons.
Signs and symptoms of unruptured cerebral aneurysms include:
- Headache
- Eye pain
- Unilateral complete third cranial nerve palsy
- Ischemic or embolic cerebrovascular disease
- Seizures
- Visual loss or hemianopsia [6]
Signs and symptoms of ruptured cerebral aneurysms include:
- Headache
- Nausea and vomiting
- Nuchal rigidity
- Altered mental status
- Eye pain
- Unilateral complete third cranial nerve palsy
- Loss of consciousness
- Photophobia
- Focal neurologic deficits
- Coma and death [19]
Headache, described as the "worst headache of my life," is the most common symptom of a ruptured cerebral aneurysm. About 10% to 43% of patients with subarachnoid hemorrhage (SAH) experience mild-to-moderate headaches approximately 2 months before aneurysm rupture, and 30% to 50% of patients report a sudden onset headache 6 to 20 days before rupture. This headache could be a warning sign of an impending rupture. Approximately 10% to 30% of patients with aneurysm rupture die before reaching the hospital, another 30% die in the hospital or are severely disabled when discharged, and only 30% have a good recovery after appropriate treatment.[4]
Hunt and Hess Classification System
This system classifies the severity of SAH based on clinical findings and predicts outcomes and mortality.
Five different grades range in the severity of symptoms, which can correlate with the overall mortality of SAH:
- Grade I - Mild headache with slight nuchal rigidity; approximately 30% of mortality
- Grade II - Severe headache with full nuchal rigidity and no neurologic deficits other than cranial nerve palsy; approximately 40% of mortality
- Grade III - Drowsiness or confusion with a mild focal deficit; approximately 50% of mortality
- Grade IV - Stuporous with moderate to severe hemiparesis; approximately 80% of mortality
- Grade V - Coma with decerebrate posturing; approximately 90% of mortality [20]
The Modified Fisher Classification
Describes the amount of blood seen on a head non-contrast computed tomography (CT) scan to predict the likelihood of developing vasospasm, ischemia, and stroke.[21] This classification was adapted from the original Fisher classification:
- Fisher 0: No blood detected
- The incidence of symptomatic vasospasm is 0%.
- Fisher 1: Thin focal or diffuse SAH.
- The incidence of symptomatic vasospasm is 18%.
- Fisher 2: Thin focal or diffuse SAH with intraventricular hemorrhage.
- The incidence of symptomatic vasospasm is 35%.
- Fisher 3: Thick focal or diffuse SAH with no intraventricular hemorrhage.
- The incidence of symptomatic vasospasm is 31%.
- Fisher 4: Thick focal or diffuse SAH with intraventricular hemorrhage.
- The incidence of symptomatic vasospasm is 68%.[22]
Original World Federation of Neurosurgical Societies Grading Scale
- Grade I: Glasgow Coma Scale Score (GCS) 15
- Grade II: GCS 13 to 14, without focal neurological deficit
- Grade III: GCS 13 to 14, with focal neurological deficit
- Grade IV: GCS 7 to 12
- Grade V: GCS 3 to 6
Modified WFNS Grading Scale
- Grade I: GCS 15
- Grade II: GCS 14
- Grade III: GCS 13
- Grade IV: GCS 7 to 12
- Grade V: GCS 3 to 6 [23]
Saccular Aortic Aneurysms
Most people with aortic aneurysms are asymptomatic. Symptoms develop as the aneurysm enlarges and creates pressure on surrounding structures.
Signs and symptoms of unruptured aortic aneurysms include:
- Chest pain or tenderness
- Neck pain
- Cough
- Shortness of breath
- Wheezing
- Hoarseness
- Dysphagia
- Abdominal pain
- Back pain
- Trouble swallowing
Signs and symptoms of ruptured aortic aneurysms include:
- Sudden, intense, and persistent chest pain that radiates to the back or persistent upper back pain
- Sudden, intense, and persistent abdominal pain
- Difficult breathing
- Low blood pressure
- Paralysis or weakness of lower extremities
- Loss of consciousness
- Trouble swallowing
- Sudden cardiac death
Evaluation
Cerebral Aneurysms
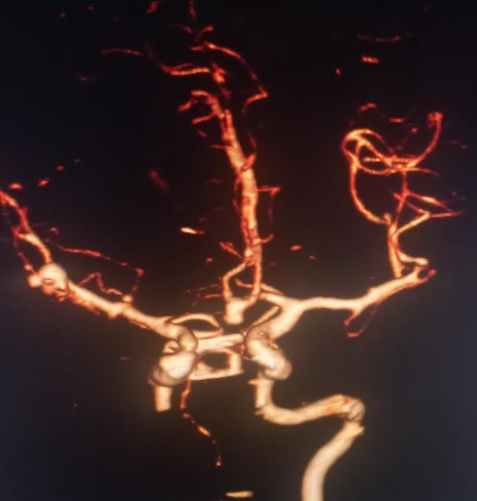
The majority of unruptured cerebral aneurysms are found incidentally during brain imaging. High-risk individuals like those with a family history of cerebral aneurysms or those with medical conditions associated with cerebral aneurysms may be screened with magnetic reasoning angiography, computerized tomography (CT) angiography (CTA) (see Image. Basilar Tip Saccular Aneurysm on 3D CTA), or digital subtraction angiography. Given the inflammatory changes and macrophage deposition occurring at the aneurysm wall, magnetic resonance imaging (MRI) with contrast may be utilized to detect aneurysms prone to rupture.[16]
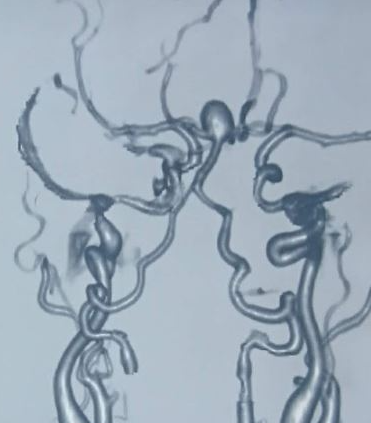
Ferumoxytol is a nanoparticle cleared by macrophages and can be used as a contrast agent for detecting inflammation.[16] A head CT scan without contrast is the initial test for a suspected ruptured cerebral aneurysm causing SAH. This is 100% sensitive for SAH if done within 6 hours of symptoms onset, but sensitivity decreases to 95% in 12 hours, 92% in 24 hours, and 50% in 1 week. If clinical suspicion is high and the head CT scan is negative, a lumbar puncture is indicated to measure cerebrospinal fluid's (CSF) cell count and xanthochromia. Due to hemoglobin breakdown products, CSF xanthochromia may take 4 hours to develop, but it is almost 100% sensitive between 12 hours and 1 week. After the diagnosis of SAH is confirmed, the source of bleeding can be identified with either CTA, MRA (see Image. Multiple Intracranial Saccular Aneurysms) or digital subtraction angiography (DSA). DSA is considered the gold standard for the diagnosis of SAH due to cerebral aneurysm rupture.
Aortic Aneurysms
Like cerebral aneurysms, they are usually found incidentally during imaging. Chest x-ray (CXR) most commonly identifies asymptomatic aortic aneurysms. CXR usually shows mediastinal widening, aortic knob enlargement, or tracheal deviation. The image may also show the displacement of aortic calcifications, aortic kinking, and aortopulmonary window opacification. Aortic aneurysms may also be incidentally discovered with an echocardiogram, chest CT scan, or chest MRI.
In symptomatic patients, CTA or magnetic reasoning angiography is the imaging of choice for better characterization of aortic diameter and vessel anatomy and to identify aortic dissection or aneurysm rupture. The choice of imaging technique depends on the clinical circumstances. CTA is a better choice in highly symptomatic patients as it is readily available and requires much less time to perform. Magnetic reasoning angiography is better than CTA in aneurysms involving the aortic root sinus due to the lack of ionizing radiation for patients requiring repeated imaging. Transthoracic echocardiography should be performed in patients with a bicuspid aortic valve to assess the diameter of an aortic sinus. Transesophageal echocardiography is preferred over transthoracic echocardiography for evaluating the entire aorta.
Treatment / Management
Cerebral Aneurysms
When deciding how to treat cerebral aneurysms, it is important to consider the patient's age and medical conditions, the size and location of the aneurysm, the presence or absence of symptoms, other risk factors for aneurysm rupture, and if an SAH has occurred. Three main strategies are utilized for the management of cerebral aneurysms: observation, endovascular therapy, and surgical therapy. All ruptured aneurysms should undergo endovascular or surgical therapy.
Observation
Patients with an unruptured cerebral aneurysm in the anterior circulation, who are older than 64 years, have an aneurysm size less than 7 mm, are asymptomatic, have no history of a SAH, or have no family history of SAH can be observed with clinical and radiological monitoring. Serial aneurysm monitoring can be achieved with CTA or MRA. Literature suggests that the annual risk of aneurysm rupture is approximately 1%.[6] Recently, some studies have evaluated the use of nonsteroidal anti-inflammatory drugs and selective COX-2 inhibitors to inhibit key inflammatory mediators involved in the growth of unruptured aneurysms.[24][25][26]
Endovascular Embolization
A minimally invasive technique in which a catheter is advanced from the femoral artery into the cerebral aneurysm. Platinum coils are inserted into the aneurysm using a second microcatheter. The coiled aneurysm forms a clot (embolization) and obliterates the aneurysm sac, preventing future rupture. The procedure can be performed under local or general anesthesia, although the latter is preferred. This procedure can treat aneurysms that are difficult to approach surgically. Flow diversion devices have recently been used to reduce blood flow inside the aneurysm, ultimately thrombosing the sac and creating a new endothelium at the neck. A new treatment with a flow disruption device is currently used; the self-expanding mesh device is placed inside the aneurysm to prevent blood flow into the sac.
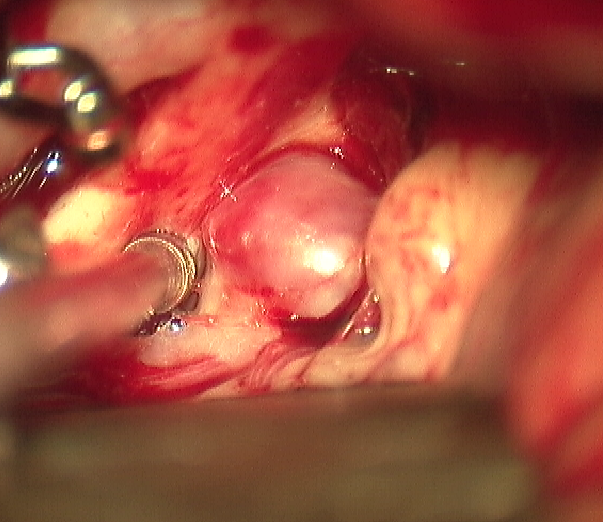
Surgical Clipping
This procedure is performed in the operating room under general anesthesia and involves making an opening of the skull (craniotomy) and dissection through the brain cisterns and fissures to expose the aneurysm (see Image. Saccular Anterior Communicating Artery Aneurysm (ACOM) Visualized Intraoperatively). After the aneurysm is exposed, a metal clip is placed across the neck to prevent blood flow into the aneurysm sac.[27][28] A disadvantage of this procedure includes its invasiveness. The annual risk of post-surgical rebleeding ranges from 0.0% to 0.9%, unlike endovascular coiling, which is 2.6%--but the morbidity and mortality associated with the surgery are higher.[6]
Patients with a ruptured cerebral aneurysm should be admitted to the intensive care unit for close monitoring. Endovascular coiling or surgical clipping of the ruptured aneurysm should be performed as early as possible in the majority of cases to reduce the risk of rebleeding. Patients need to be monitored for the signs and symptoms of clinical deterioration due to vasospasm, rebleeding, seizures, cerebral edema, hydrocephalus, and hyponatremia. In the first 48 hours after SAH, the major cause of morbidity or mortality is rebleeding. Blood pressure should be maintained near 120/80 mm Hg before the definite procedure to occlude the aneurysm; after treatment, the mean arterial pressure should be maintained above 100 mm Hg to reduce ischemic changes secondary to vasospasm. Cerebral vasospasm resulting in cerebral ischemic and neurologic deterioration starts around day 3 to 5 and peaks at day 7. Oral nimodipine at a dose of 60 mg every 4 hours is given for 21 days to improve outcomes.[29][30] Daily transcranial Doppler is recommended to monitor vasospasm. After aneurysm repair, cerebrovascular imaging is generally recommended to look for remnants or recurrence of the aneurysm that may require re-intervention. This is performed intraoperatively or a few days later.
Aortic Aneurysms
Treatment modalities depend on size, symptoms, location, and other comorbidities. The most important determinant for rupture is the diameter of the aneurysm. The location of the aneurysm, the presence of coronary artery disease, and aortic valve pathology dictate the type of repair to be performed.
Conservative management
Patients who are asymptomatic with an aneurysm diameter <45 mm are managed with medical therapy and active surveillance.[13] Saccular aortic aneurysms enlarge at a rate of 2.9 mm/year.[3] This growth is similar to that of fusiform aneurysms. Conservative management includes aggressive blood pressure control (goal <130/80 mm Hg), screening for medical conditions associated with aortic aneurysms, avoidance of fluoroquinolones, cardiovascular risk reduction measures, and serial imaging annually or biannually depending on the diameter of the aorta to evaluate changes in aortic diameter. Beta-blockers are the preferred blood pressure medications. Saccular aortic aneurysms become symptomatic or acute at smaller diameters than fusiform aneurysms, supporting the idea that saccular aneurysms should be treated at smaller diameters than fusiform aneurysms.[13]
Repair management
All symptomatic unruptured or ruptured saccular aortic aneurysms should undergo repair. The exact diameter threshold for elective treatment of asymptomatic aneurysms is challenging to determine, but a diameter of 45 mm seems to be an acceptable threshold.[13] A smaller diameter is suggested to indicate repair in asymptomatic patients with genetically mediated aneurysms. Asymptomatic aneurysms with expansion greater or equal to 5 mm per year should undergo repair. Patients undergoing coronary artery bypass or aortic valve surgery with ascending aorta aneurysms and diameter >45 mm should also have repair. Open repair is indicated for aneurysms that involve the aortic arch, ascending aorta, and aortic root. Endovascular aneurysm repair is preferred in patients with descending aortic aneurysms.
Differential Diagnosis
Cerebral Aneurysms
- Intracerebral hematoma
- Subdural hematoma
- Cerebral stroke
- Intracranial arterial dissection
- Mycotic aneurysm
- Arteriovenous malformations
- Cerebral amyloid angiopathy
- Reversible cerebral vasoconstriction syndrome
- Vasculitis
- Dural sinus thrombosis
- Cerebral venous thrombosis
Aortic Aneurysms
- Aortic dissection
- Aortic pseudoaneurysm
- Senile ectasia
- Post-stenotic dilatation
- Acute myocardial infarction
- Pneumothorax
- Superior vena cava syndrome
- Pulmonary embolism
- Hypertensive emergency
- Mediastinal mass
- Lung mass abutting aorta
- Abdominal mass
- Mesenteric ischemia
- Diverticulitis
- Pyelonephritis
Prognosis
Small unruptured cerebral aneurysms (less than 7 mm) have a low risk of rupture. Morbidity and mortality are high when a cerebral aneurysm ruptures. SAH due to aneurysm rupture is a catastrophic event with a mortality rate ranging from 25% to 50%. Nearly 50% of the survivors will have a permanent disability.[16] Approximately one-third of patients have a good outcome after appropriate treatment. Cerebral aneurysms can also bleed into the brain parenchyma, causing intraparenchymal hemorrhage and adding extra insult to the brain. The result of a ruptured cerebral aneurysm depends on the extent of the bleed, the location of the bleed, the age of the patient, neurological status on presentation, the degree of vasospasm, and associated comorbidities.
The annual risk of rupture of an aortic aneurysm is low when the size is less than 45 mm. When an aneurysm ruptures, approximately 50% of patients die before they reach the emergency department. Those who survive have very high morbidity. When all locations are included, approximately 2% to 17% perioperative mortality is expected, with the arch of the aorta location approaching a 25% mortality. The outcome depends on the timing of intervention, the location, the type of surgery, the experience of the surgeon, concomitant comorbidities, and if it has ruptured.
Complications
Cerebral Aneurysms
- Cerebral aneurysm rupture
- Recurrent bleeding
- Vasospasm
- Seizures
- Cerebral salt wasting
- Syndrome of inappropriate antidiuretic hormone secretion
- Hydrocephalus
- Stunted myocardium and pulmonary edema
- Arrhythmias
- Gastrointestinal bleeding
- Infections
- Deep venous thrombosis
- Death
Aortic Aneurysms
- Aortic aneurysm rupture
- Acute myocardial infarction
- Aortic regurgitation
- Superior vena cava syndrome or thromboembolism
- Pneumonitis
- Heart failure
- Hemodiaphragm paralysis due to phrenic nerve compression
- Aortoesophageal fistula
- Death
Postoperative and Rehabilitation Care
Cerebral Aneurysms
- After discharge, it is reasonable to refer most patients for a comprehensive evaluation, including cognitive, behavioral, psychosocial, physical, speech, and occupational therapy evaluation.
- Tobacco and alcohol counseling should be provided if they have these behaviors.
- Provide deep venous thrombosis prophylaxis if they have minimal or nonambulatory status.
- Use serial imaging to monitor the aneurysm's complete obliteration after repair or the aneurysm size if not occluded.
Aortic Aneurysms
- Cardiac rehabilitation program
- Post-discharge close follow-up
- Aggressive blood pressure control
- Cardiovascular risk reduction measures
Consultations
The following consultations are recommended:
- Neurosurgeon
- Critical care specialist
- Interventional neuroradiologist
- Vascular interventional radiologist
- Vascular surgeon
- Cardiothoracic surgeon
- Neurologist
- Rehabilitation team
Deterrence and Patient Education
Patients should be educated about cerebral and aortic aneurysms and the risks associated with them. A ruptured aneurysm is a serious and life-threatening emergency condition. Approximately 10% to 30% of the patients with a cerebral aneurysm bleed before reaching the hospital, and only about 30% recover after appropriate treatment. Tobacco smoking and excessive alcohol consumption should be avoided as this can increase the risk of aneurysm progression or rupture. Better blood pressure control is recommended as this is one of the risk factors for aneurysm formation, progression, and rupture. Cardiovascular risk reduction measures should be implemented.
Pearls and Other Issues
Below are some of the important pearls related to saccular aneurysms:
- Early treatment of ruptured aneurysms reduces the rate of rebleeding and facilitates the treatment of vasospasm.
- Noninvasive studies may be indicated in patients with conditions associated with cerebral aneurysms and those with a family history of SAH.
- Cardiologist consultation may be used as these patients can develop stunted myocardium and pulmonary edema.
- A nephrologist may be needed as these patients are at risk for electrolyte abnormalities.
Enhancing Healthcare Team Outcomes
An interprofessional approach is vital for better clinical outcomes. Early diagnosis and referral to a neurosurgeon, vascular surgeon, or interventional radiologist have better outcomes in patients with ruptured saccular aneurysms. An interprofessional team including but not limited to an emergency medicine clinician, a neurosurgeon, an endovascular interventional neuroradiologist, an intensivist, a vascular surgeon, a cardiothoracic surgeon, a neurologist, a cardiologist, a physical therapist, a speech therapist, an occupational therapist, a nurse, a pharmacist, and a nephrologist is recommended.