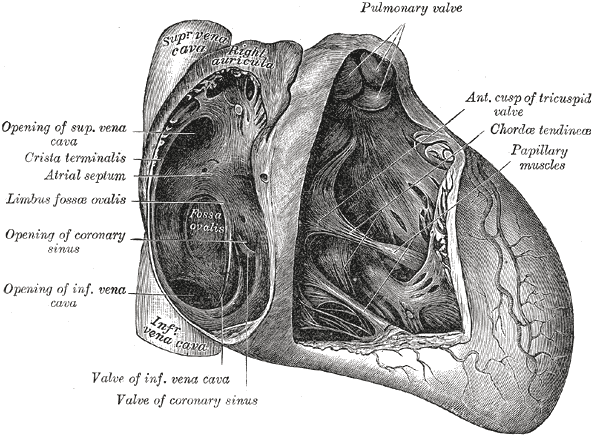
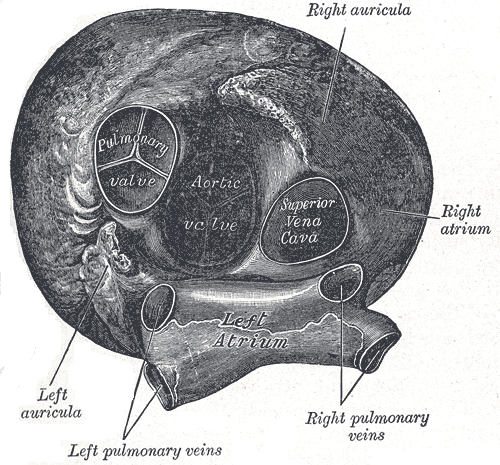
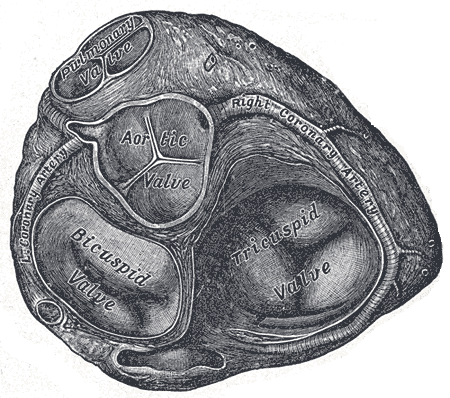
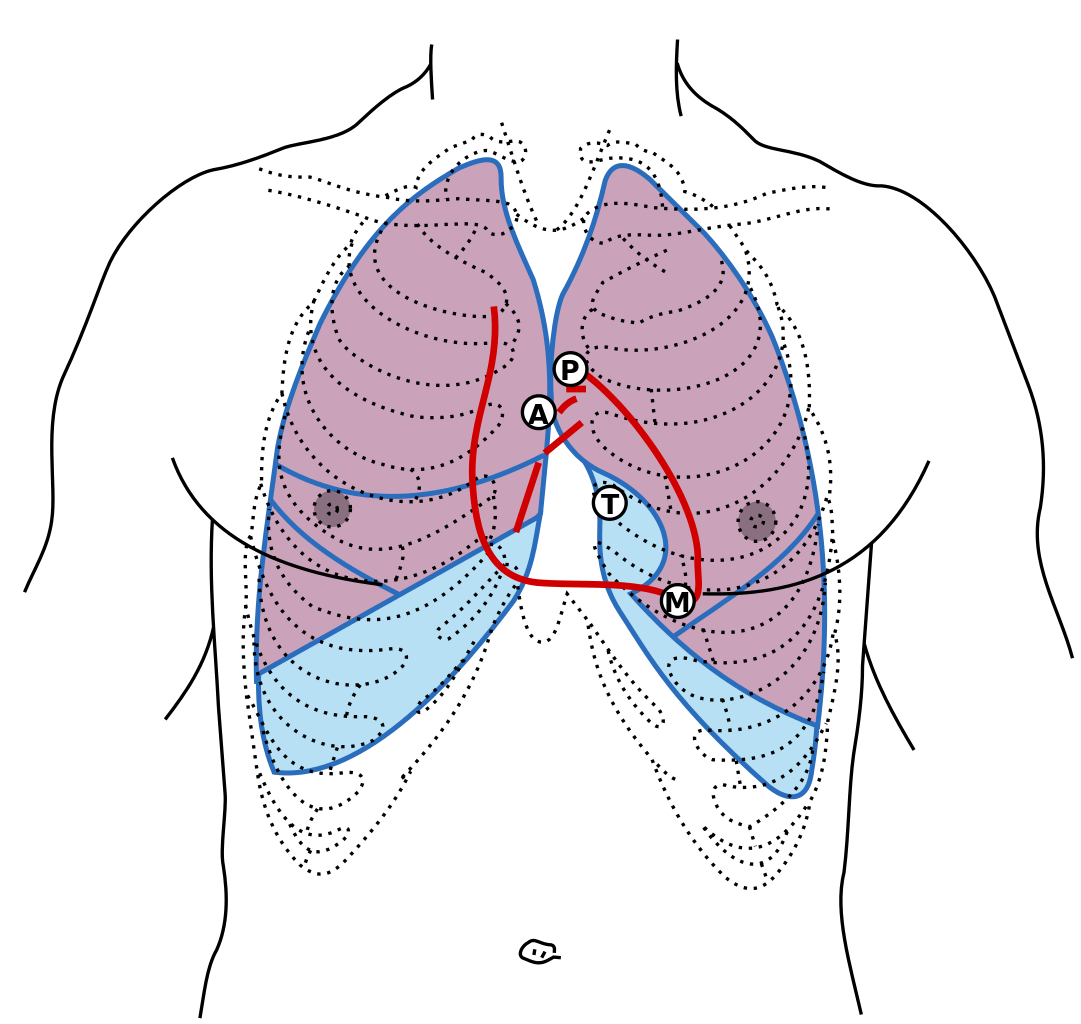
[1]
Ho SY, Nihoyannopoulos P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart (British Cardiac Society). 2006 Apr:92 Suppl 1(Suppl 1):i2-13
[PubMed PMID: 16543598]
[2]
Stephens EH, Kearney DL, Grande-Allen KJ. Insight into pathologic abnormalities in congenital semilunar valve disease based on advances in understanding normal valve microstructure and extracellular matrix. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2012 Jan-Feb:21(1):46-58. doi: 10.1016/j.carpath.2011.01.002. Epub 2011 Feb 23
[PubMed PMID: 21349746]
Level 3 (low-level) evidence
[3]
Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circulation research. 2009 Aug 28:105(5):408-21. doi: 10.1161/CIRCRESAHA.109.201566. Epub
[PubMed PMID: 19713546]
[4]
Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008 Oct 28:118(18):1864-80. doi: 10.1161/CIRCULATIONAHA.108.805911. Epub
[PubMed PMID: 18955677]
[5]
Harper WF. The blood supply of heart valves in relation to endocarditis. Journal of anatomy. 1938 Oct:73(Pt 1):94-111
[PubMed PMID: 17104751]
[7]
Ratajska A, Gula G, Flaht-Zabost A, Czarnowska E, Ciszek B, Jankowska-Steifer E, Niderla-Bielinska J, Radomska-Lesniewska D. Comparative and developmental anatomy of cardiac lymphatics. TheScientificWorldJournal. 2014:2014():183170. doi: 10.1155/2014/183170. Epub 2014 Jan 27
[PubMed PMID: 24592145]
Level 2 (mid-level) evidence
[8]
Marron K, Yacoub MH, Polak JM, Sheppard MN, Fagan D, Whitehead BF, de Leval MR, Anderson RH, Wharton J. Innervation of human atrioventricular and arterial valves. Circulation. 1996 Aug 1:94(3):368-75
[PubMed PMID: 8759078]
[9]
Anderson RH, Razavi R, Taylor AM. Cardiac anatomy revisited. Journal of anatomy. 2004 Sep:205(3):159-77
[PubMed PMID: 15379923]
[11]
Ross DN. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet (London, England). 1967 Nov 4:2(7523):956-8
[PubMed PMID: 4167516]
[12]
Stulak JM, Burkhart HM, Sundt TM 3rd, Connolly HM, Suri RM, Schaff HV, Dearani JA. Spectrum and outcome of reoperations after the Ross procedure. Circulation. 2010 Sep 21:122(12):1153-8. doi: 10.1161/CIRCULATIONAHA.109.897538. Epub 2010 Sep 7
[PubMed PMID: 20823390]
[13]
Alsoufi B, Al-Halees Z, Manlhiot C, McCrindle BW, Al-Ahmadi M, Sallehuddin A, Canver CC, Bulbul Z, Joufan M, Fadel B. Mechanical valves versus the Ross procedure for aortic valve replacement in children: propensity-adjusted comparison of long-term outcomes. The Journal of thoracic and cardiovascular surgery. 2009 Feb:137(2):362-370.e9. doi: 10.1016/j.jtcvs.2008.10.010. Epub
[PubMed PMID: 19185153]
[14]
Mastrobuoni S, de Kerchove L, Solari S, Astarci P, Poncelet A, Noirhomme P, Rubay J, El Khoury G. The Ross procedure in young adults: over 20 years of experience in our Institution. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2016 Feb:49(2):507-12; discussion 512-3. doi: 10.1093/ejcts/ezv053. Epub 2015 Mar 3
[PubMed PMID: 25736279]
[15]
Ungerleider RM, Ootaki Y, Shen I, Welke KF. Modified Ross procedure to prevent autograft dilatation. The Annals of thoracic surgery. 2010 Sep:90(3):1035-7; discussion 1037. doi: 10.1016/j.athoracsur.2009.09.078. Epub
[PubMed PMID: 20732551]
[16]
Stelzer P, Weinrauch S, Tranbaugh RF. Ten years of experience with the modified Ross procedure. The Journal of thoracic and cardiovascular surgery. 1998 May:115(5):1091-100
[PubMed PMID: 9605079]
[17]
FORD AB, HELLERSTEIN HK, WOOD C, KELLY HB. Isolated congenital bicuspid pulmonary valve; clinical and pathologic study. The American journal of medicine. 1956 Mar:20(3):474-86
[PubMed PMID: 13292455]
[18]
Angeli E, Gerelli S, Beyler C, Lamerain M, Rochas B, Bonnet D, Vouhé P, Raisky O. Bicuspid pulmonary valve in transposition of the great arteries: impact on outcome. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012 Feb:41(2):248-55. doi: 10.1016/j.ejcts.2011.03.063. Epub 2011 Dec 12
[PubMed PMID: 21757366]
[19]
Goda M, Budts W, Troost E, Meyns B. Bicuspid pulmonary valve with atrial septal defect leading to pulmonary aneurysm. The Annals of thoracic surgery. 2012 May:93(5):1706-8. doi: 10.1016/j.athoracsur.2011.09.063. Epub
[PubMed PMID: 22541203]
[20]
Jamis-Dow CA, Barbier GH, Watkins MP, Lanza GM, Caruthers SD, Wickline SA. Bicuspid Pulmonic Valve and Pulmonary Artery Aneurysm. Cardiology research. 2014 Apr:5(2):83-84
[PubMed PMID: 26191115]
[21]
Manivarmane R, Taylor R, Khattar R. A case of isolated bicuspid pulmonary valve. Echo research and practice. 2017 Dec 12:5(1):K13-8. doi: 10.1530/ERP-17-0045. Epub 2017 Dec 12
[PubMed PMID: 29233813]
Level 3 (low-level) evidence
[22]
Vedanthan R, Sanz J, Halperin J. Bicuspid pulmonic valve. Journal of the American College of Cardiology. 2009 Aug 18:54(8):e5. doi: 10.1016/j.jacc.2009.05.027. Epub
[PubMed PMID: 19679243]
[24]
Ascione L, lengo R, Tuccillo B, D'Andrea A, De Michele M, Porto A, Ronza F, Del Viscovo L. Quadricuspid pulmonary valve diagnosed by cardiac magnetic resonance. Journal of cardiovascular medicine (Hagerstown, Md.). 2009 Dec:10(12):944-5
[PubMed PMID: 20054880]
[25]
Akerem Khan SK, Anavekar NS, Araoz PA. Quadricuspid pulmonary valve: computed tomography case series and review of relevant literature. Journal of thoracic imaging. 2012 Nov:27(6):W171-3. doi: 10.1097/RTI.0b013e31822e864c. Epub
[PubMed PMID: 23090364]
Level 2 (mid-level) evidence
[26]
Hurwitz LE, Roberts WC. Quadricuspid semilunar valve. The American journal of cardiology. 1973 May:31(5):623-6
[PubMed PMID: 4698133]
[27]
Dunay SN, Roberge RA, Avedissian LS. Quadricuspid pulmonic valve found on well exam. Military Medical Research. 2015:2():10. doi: 10.1186/s40779-015-0037-2. Epub 2015 Apr 23
[PubMed PMID: 25922689]
[28]
Demircin S, Keles-Coskun N. A case of pentacuspid pulmonary valve. Surgical and radiologic anatomy : SRA. 2010 Jul:32(6):613-5. doi: 10.1007/s00276-009-0607-7. Epub 2009 Dec 19
[PubMed PMID: 20024548]
Level 3 (low-level) evidence
[29]
Kadowaki T, Yamaguchi H, Takagaki M, Nakamura H, Mitsuyama S, Ando T, Takinami G, Nakao T. Successful Repair of Regurgitant Pentacuspid Pulmonary Valve Combined With Pulmonary Artery Aneurysm. The Annals of thoracic surgery. 2016 May:101(5):1990-2. doi: 10.1016/j.athoracsur.2015.07.038. Epub
[PubMed PMID: 27106440]
[30]
Samánek M, Slavík Z, Zborilová B, Hrobonová V, Vorísková M, Skovránek J. Prevalence, treatment, and outcome of heart disease in live-born children: a prospective analysis of 91,823 live-born children. Pediatric cardiology. 1989 Fall:10(4):205-11
[PubMed PMID: 2687820]
[31]
Stephensen SS, Sigfusson G, Eiriksson H, Sverrisson JT, Torfason B, Haraldsson A, Helgason H. Congenital cardiac malformations in Iceland from 1990 through 1999. Cardiology in the young. 2004 Aug:14(4):396-401
[PubMed PMID: 15680046]
[33]
Way RC. Cardiovascular defects and the rubella syndrome. Canadian Medical Association journal. 1967 Nov 25:97(22):1329-34
[PubMed PMID: 6061597]
[34]
Ko JM. Genetic Syndromes associated with Congenital Heart Disease. Korean circulation journal. 2015 Sep:45(5):357-61. doi: 10.4070/kcj.2015.45.5.357. Epub 2015 Jul 7
[PubMed PMID: 26413101]
[35]
Bhattacharyya S, Davar J, Dreyfus G, Caplin ME. Carcinoid heart disease. Circulation. 2007 Dec 11:116(24):2860-5
[PubMed PMID: 18071089]
[36]
Xanthos T, Dalivigkas I, Ekmektzoglou KA. Anatomic variations of the cardiac valves and papillary muscles of the right heart. Italian journal of anatomy and embryology = Archivio italiano di anatomia ed embriologia. 2011:116(2):111-26
[PubMed PMID: 22303639]
[37]
Altun G, Babaoğlu K, Binnetoğlu K, Kavas N, Arısoy AE. Functional pulmonary atresia in newborn with normal intracardiac anatomy: Successful treatment with inhaled nitric oxide and pulmonary vasodilators. Annals of pediatric cardiology. 2013 Jan:6(1):83-6. doi: 10.4103/0974-2069.107243. Epub
[PubMed PMID: 23626445]
[38]
Lancellotti P, Tribouilloy C, Hagendorff A, Moura L, Popescu BA, Agricola E, Monin JL, Pierard LA, Badano L, Zamorano JL, European Association of Echocardiography. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: aortic and pulmonary regurgitation (native valve disease). European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2010 Apr:11(3):223-44. doi: 10.1093/ejechocard/jeq030. Epub
[PubMed PMID: 20375260]
[39]
Chaturvedi RR, Redington AN. Pulmonary regurgitation in congenital heart disease. Heart (British Cardiac Society). 2007 Jul:93(7):880-9
[PubMed PMID: 17569817]
[40]
Shimazaki Y, Blackstone EH, Kirklin JW. The natural history of isolated congenital pulmonary valve incompetence: surgical implications. The Thoracic and cardiovascular surgeon. 1984 Aug:32(4):257-9
[PubMed PMID: 6207619]
[41]
Tariq M, Smego RA Jr, Soofi A, Islam N. Pulmonic valve endocarditis. Southern medical journal. 2003 Jun:96(6):621-3
[PubMed PMID: 12938795]
[42]
Kang N, Smith W, Greaves S, Haydock D. Pulmonary-valve endocarditis. The New England journal of medicine. 2007 May 24:356(21):2224-5
[PubMed PMID: 17522411]
[43]
Seraj SM, Gill E, Sekhon S. Isolated pulmonary valve endocarditis: truth or myth? Journal of community hospital internal medicine perspectives. 2017:7(5):329-331. doi: 10.1080/20009666.2017.1374808. Epub 2017 Oct 18
[PubMed PMID: 29147479]
Level 3 (low-level) evidence
[44]
Swaminath D, Yaqub Y, Narayanan R, Paone RF, Nugent K, Arvandi A. Isolated Pulmonary Valve Endocarditis Complicated With Septic Emboli to the Lung Causing Pneumothorax, Pneumonia, and Sepsis in an Intravenous Drug Abuser. Journal of investigative medicine high impact case reports. 2013 Oct-Dec:1(4):2324709613514566. doi: 10.1177/2324709613514566. Epub 2013 Nov 28
[PubMed PMID: 26425590]
Level 3 (low-level) evidence
[45]
Ranjith MP, Rajesh KF, Rajesh G, Haridasan V, Bastian C, Sajeev CG, Krishnan MN. Isolated pulmonary valve endocarditis: A case report and review of literature. Journal of cardiology cases. 2013 Nov:8(5):161-163. doi: 10.1016/j.jccase.2013.07.007. Epub 2013 Sep 28
[PubMed PMID: 30534282]
Level 3 (low-level) evidence
[46]
Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015 Oct 13:132(15):1435-86. doi: 10.1161/CIR.0000000000000296. Epub 2015 Sep 15
[PubMed PMID: 26373316]
[47]
Hoen B, Béguinot I, Rabaud C, Jaussaud R, Selton-Suty C, May T, Canton P. The Duke criteria for diagnosing infective endocarditis are specific: analysis of 100 patients with acute fever or fever of unknown origin. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1996 Aug:23(2):298-302
[PubMed PMID: 8842267]
[48]
Kan JS, White RI Jr, Mitchell SE, Gardner TJ. Percutaneous balloon valvuloplasty: a new method for treating congenital pulmonary-valve stenosis. The New England journal of medicine. 1982 Aug 26:307(9):540-2
[PubMed PMID: 7099226]
[49]
Peterson C, Schilthuis JJ, Dodge-Khatami A, Hitchcock JF, Meijboom EJ, Bennink GB. Comparative long-term results of surgery versus balloon valvuloplasty for pulmonary valve stenosis in infants and children. The Annals of thoracic surgery. 2003 Oct:76(4):1078-82; discussion 1082-3
[PubMed PMID: 14529989]
Level 2 (mid-level) evidence
[50]
Merino-Ingelmo R, Santos-de Soto J, Coserria-Sánchez F, Descalzo-Señoran A, Valverde-Pérez I. Long-term results of percutaneous balloon valvuloplasty in pulmonary valve stenosis in the pediatric population. Revista espanola de cardiologia (English ed.). 2014 May:67(5):374-9. doi: 10.1016/j.rec.2013.08.020. Epub 2014 Feb 5
[PubMed PMID: 24774730]
[51]
BENTIVOGLIO LG, MARANHAO V, DOWNING DF. The electrocardiogram in pulmonary stenosis with intact septa. American heart journal. 1960 Mar:59():347-57
[PubMed PMID: 13799028]
[52]
Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M, American Society of Echocardiography, European Association of Echocardiography. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2009 Jan:22(1):1-23; quiz 101-2. doi: 10.1016/j.echo.2008.11.029. Epub
[PubMed PMID: 19130998]
[53]
Rautenburg HW, Askevold IB. [The chest X-ray in children with pulmonic stenosis and intact ventricular septum (author's transl)]. Herz. 1980 Oct:5(5):306-13
[PubMed PMID: 6450150]
[54]
Chen JT, Robinson AE, Goodrich JK, Lester RG. Uneven distribution of pulmonary blood flow between left and right lungs in isolated valvular pulmonary stenosis. The American journal of roentgenology, radium therapy, and nuclear medicine. 1969 Oct:107(2):343-50
[PubMed PMID: 5823902]
[55]
Saremi F, Gera A, Ho SY, Hijazi ZM, Sánchez-Quintana D. CT and MR imaging of the pulmonary valve. Radiographics : a review publication of the Radiological Society of North America, Inc. 2014 Jan-Feb:34(1):51-71. doi: 10.1148/rg.341135026. Epub
[PubMed PMID: 24428282]
[56]
Zhou WY, Li YY, He XQ, Wang YB. Absent Pulmonary Valve Syndrome in a Fetus: A Case Report and Literature Review. Fetal and pediatric pathology. 2019 Feb:38(1):57-62. doi: 10.1080/15513815.2018.1529066. Epub 2019 Jan 20
[PubMed PMID: 30661433]
Level 3 (low-level) evidence