Introduction
The heart has an electrical system that allows it to contract and pump blood through the body in a coordinated rhythm. Regular heartbeats occur when specialized cells in the right atrium of the heart, called the sinoatrial (SA) node, conduct an electrical signal down to the atrioventricular (AV) node which is another set of specialized cells. This electrical signal then works its way down the bundle of His and Purkinje fibers to the heart ventricles. The result is the contraction of the ventricles and pumping of blood from the heart out to the body's arteries.[1]
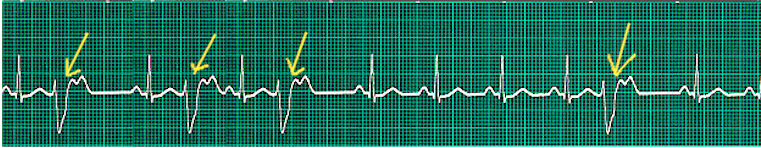
During a premature ventricular contraction (PVC), the heartbeat is initiated by the Purkinje fibers rather than the SA node. Given that a PVC occurs before a regular heartbeat, there is a pause before the next regular heartbeat.[2]
PVCs can occur in isolation or in repeated patterns. Two consecutive PVCs are termed doublets while three consecutive PVCs are named triplets. It is important to note that three or more consecutive PVCs are classified as ventricular tachycardia. If the PVCs continuously alternate with a regular sinus beat, the patient is in bigeminy.[3] Likewise, if every third heartbeat is a PVC, then it is named trigeminy.
PVCs present as heart palpitations in most patients. They are usually benign and do not require treatment.
Etiology
In the vast majority of cases, PVCs have no known cause and occur spontaneously.
Common known etiologies include excess caffeine consumption, excess catecholamines,[4] high levels of anxiety, and electrolyte abnormalities. Specific electrolyte changes found in those who experience PVCs are low blood potassium, low blood magnesium, and high blood calcium. Alcohol, tobacco, and illicit drugs are also associated with PVCs as are stimulant-based medications. Patients suffering from sleep deprivation also experience PVCs.
There are numerous cardiac and non-cardiac pathologies that are causative of PVCs. Examples include cardiomyopathy, mitral valve prolapse, and myocardial infarction. Any structural heart disease that alters conduction pathways due to tissue alterations can cause PVCs. Non-cardiac examples are hyperthyroidism, anemia, and even hypertension.
Patient populations with higher risks of cardiovascular disease and clinically poor cardiovascular markers have a higher occurrence of PVCs.[5]
Risk factors for PVC include:
- Advanced age
- Male gender
- Hypertension
- African American
- hypomagnesemia
- Bundle branch block
- Hypokalemia
- Underlying ischemic heart disease
Epidemiology
PVCs are common among the general population. The estimated prevalence ranges from 1% to 4% on electrocardiogram and 40% to 75% on a 24 or 48-hour Holter monitor.[6] Young and healthy adults have shown a highly similar frequency rate of PVCs in contrast to the older segments of the general population.
Pathophysiology
One pathophysiological reason for the occurrence of PVCs is ectopic nodal automaticity. This suggests that ectopic pacemaker cells carry a subthreshold potential for firing. If the threshold is reached via the heart's electrical activity, an ectopic beat occurs.
A second pathophysiologic explanation is re-entrant signaling. If one pathway of the Purkinje fibers is blocked and another path is experiencing slower conduction, this can trigger an ectopic beat on the post-block pathway.
The final explanation for PVCs is that triggered beats occur due to after-depolarizations.[7]
On the molecular level, there are a few changes that create an environment for spontaneous depolarization of the ventricular myocytes. These include hypokalemia, hypomagnesemia, excess calcium, and excess catecholamines.[8]
In some cases, the triggered beat may occur in patients with digoxin toxicity and following reperfusion after an MI.
History and Physical
The most common sensation associated with PVCs is that of a skipped heartbeat followed by a fluttering sensation. Patients commonly present complaining of heart palpitations. The vast majority of patients are entirely asymptomatic as there are no associated symptoms with the palpitations. Some patients may experience lightheadedness, chest pain, chest discomfort, dyspnea, and anxiety. Rarely, patients experience syncope because of PVCs.
A thorough history should include any associated symptoms with the palpitations, the patient's medical history, medication, and supplement usage as well as a detailed social history. It is crucial to inquire about any illicit drug use in those who frequently experience PVCs.
Physical examination findings would reveal an irregular heart rhythm upon auscultation if the patient is experiencing PVCs during the examination. In some patients, cannon A waves may cause chest or neck discomfort. Otherwise, there would not be any direct physical examination findings. A prolonged run of PVCs can result in hypotension.
It is important to check the patient's vitals and complete a thorough examination of their cardiovascular system. If they complain of dizziness, other potential etiologies should be investigated, and an orthostatic examination should be done.
Evaluation
The initial diagnostic test would be a 12-lead electrocardiogram to look for ectopic ventricular beats. As PVCs are infrequent in most patients, the brief period of an electrocardiogram may fail to capture the ectopic beats. This also allows the differentiation of a PVC from ectopic atrial beats, which are termed premature atrial contractions (PACs). In patients with PVCs, the ECG may reveal other findings that include:
- Electrolyte abnormalities (peaked T waves, QT prolongation)
- Left ventricular hypertrophy
- With an old MI, one may see Q waves, loss of R waves, and/or a bundle branch block
- Acute ischemia may present with ST-segment elevation/depression and/or T wave inversion
To diagnose the palpitations, a 24 or 48-hour Holter monitor may be used to differentiate the PVCs from other potential arrhythmias. It is also imperative to quantify the PVCs over a set time span to assess the patient's risk for developing cardiomyopathy. Event monitors can be used for the same purpose, particularly if the patient's complaints are infrequent, as they are done up to 30 days.
Laboratory testing is also essential, particularly in identifying the potential etiology of the PVCs. A complete blood count, potassium, calcium, magnesium, and thyroid-stimulating hormone should be ordered.
Further diagnostic testing options include echocardiography and stress testing. The value of an echocardiogram for the evaluation of PVCs is to rule out structural heart disease, but only if there is clinical suspicion. Stress testing is another part of a thorough cardiac evaluation. It holds diagnostic value for those who experience PVCs during exercise or in the recovery phase following exercise.
The Lown Grading system for PVCs
- Grade 0: No evidence of premature beats
- Grade 1: Occasional PVCs (less than 30/hour)
- Grade 2: Frequent PVCs (more than 30/hour)
- Grade 3: Multiform
- Grade 4: Repetitive PVCs (A-couplets, B-salvos of more than 3)
- Grade 5: R on T pattern
Treatment / Management
Patients who experience asymptomatic PVCs rarely require any treatment. This is especially true for isolated PVCs. In the emergency room, hypoxic patients need to be provided with oxygen, the electrolyte imbalance should be corrected and drug toxicity should be ruled out. At the same time, an acute MI must be ruled out.
Routine use of lidocaine and other antiarrhythmic medications in the presence of an acute MI is not recommended.
Those experiencing frequent PVCs or symptomatic PVCs should be evaluated to identify the etiology. In many cases, excess intake of stimulants and/or lower levels of potassium and magnesium is the cause of the PVCs. These patients can be easily managed via minimization of stimulants and/or repletion of electrolytes. As well, these management options are viable for those who have asymptomatic and infrequent PVCs but still find them bothersome. Both types of patients also benefit from the minimization of stress.
The medication classes used to treat frequent and/or symptomatic PVCs include antiarrhythmics, beta-blockers, and calcium channel blockers. Commonly used antiarrhythmics include amiodarone and flecainide.
Some patients who have very frequent PVCs (e.g., several thousand per day) or symptomatic PVCs refractory to pharmacologic treatment, may be candidates for radiofrequency catheter ablation. This type of procedure is performed by an electrophysiologist who will eliminate the specific area of heart tissue that is causing the ectopic beats. Successful treatment has been shown to reverse PVC-induced cardiomyopathy.[9][10][11]
Differential Diagnosis
- PAC - Often have a very similar sensation to PVCs but are benign. A PAC also has a shorter compensatory pause than a PVC.
- Non-sustained ventricular tachycardia (NSVT) - Three or more consecutive PVCs are defined as NSVT until a time length of 30 seconds.
- Sustained ventricular tachycardia
- Bigeminy
- Trigeminy
- Quadrigeminy
- Supraventricular tachycardia
- Sinus arrhythmia
- Atrial fibrillation
Prognosis
Healthy patients without structural heart disease who experience infrequent PVCs have the same prognosis as the general population. However, those with frequent PVCs (> 1000/day) are at risk of developing dilated cardiomyopathy.[12]
For those with heart disease, PVCs are an indicator of increased mortality risk.[13]
PVCs occurring during a period of exercise have a varying prognosis, depending on when they occur. If the PVCs occur during exercise, the prognosis is very good. If they occur during the recovery phase of exercise, the long-term risk of mortality is higher than the general population.[14]
In general, PVCs that occur in patients with left ventricular dysfunction are associated with high mortality. In addition, PVCs that are not inducible during an EP study is of low risk for sudden death.
There are major limitations in the literature for studying the prognosis of PVCs. Many patients have subclinical heart disease or long-term risk factors that alter prognosis.
Enhancing Healthcare Team Outcomes
PVCs may present at any time and in any patient, thus, it is important for the interprofessional team of clinician and nurse to be aware of them and react to them appropriately. However, it is the ICU or cardiology nurse that will most likely be the first person to observe PVCs on the monitor or ECG strip. For patients without symptoms, the prognosis is excellent. However, asymptomatic patients with an ejection fraction of less than 40% may have a slightly higher risk of developing ventricular arrhythmias and even cardiac arrest. However, in patients with no evidence of heart anomalies, all that is required is reassurance. Recent data seem to suggest that very frequent PVCs may be associated with the development of cardiomyopathy but more research is required before any interventions can be recommended. In patients who suffer a myocardial infarction and develop PVCs, the risk of degeneration into a serious ventricular arrhythmia is very rare. However, if the ectopic beats persist, then the patient should be referred to an electrophysiologist. When clinicians detect PVCs it is important to discuss with a cardiologist their clinical significance. Only through open communication can the outcomes of patients with PVCs be improved.
Overall, the presence of PVCs in young people is a benign finding but in older patients with underlying heart disease, there is a risk of ventricular arrhythmias and cardiac arrest.[15][16] [Level 5]