Continuing Education Activity
Papillary muscle rupture is a rare but potentially fatal complication that typically follows a myocardial infarction or occurs secondary to infective endocarditis. Acute rupture frequently results in severe mitral valve regurgitation, acute life-threatening cardiogenic shock, and pulmonary edema. Papillary muscle dysfunction leads to regurgitation of blood through the valves, causing the backward flow of blood and, potentially, left- or right-sided heart failure.
Diagnosis of papillary muscle rupture relies primarily on echocardiography, which may reveal ruptured or dysfunctional papillary muscles, severe mitral regurgitation, and signs of hemodynamic instability. Management requires urgent stabilization, often with vasopressors or intraaortic balloon pump support, followed by prompt surgical intervention, typically mitral valve repair or replacement.
The prognosis is generally poor without immediate treatment due to the high risk of cardiogenic shock and other complications. Mortality remains significant even with surgical intervention, particularly in patients of advanced age or with severe comorbidities. Early detection and rapid intervention improve survival outcomes, but long-term prognosis depends on the extent of left ventricular damage and overall patient health.
This activity for healthcare professionals is designed to enhance learner's competence in evaluating and managing papillary muscle rupture. Participants gain a deeper understanding of the condition's pathophysiology, clinical presentation, and evidence-based diagnostic and treatment strategies. Improved proficiency enables clinicians to collaborate within an interprofessional team caring for affected patients.
Objectives:
Identify the clinical features of papillary muscle rupture.
Apply clinically guided diagnostic approaches for papillary muscle rupture.
Implement personalized, evidence-based management strategies for papillary muscle rupture.
Collaborate with other healthcare professionals to manage papillary muscle rupture and coordinate a comprehensive cardiovascular care plan.
Introduction
Papillary muscle rupture is a rare but potentially fatal complication, typically occurring after myocardial infarction or due to infective endocarditis. Acute rupture often results in severe mitral valve regurgitation, leading to acute life-threatening cardiogenic shock and pulmonary edema.[1][2][3][4]
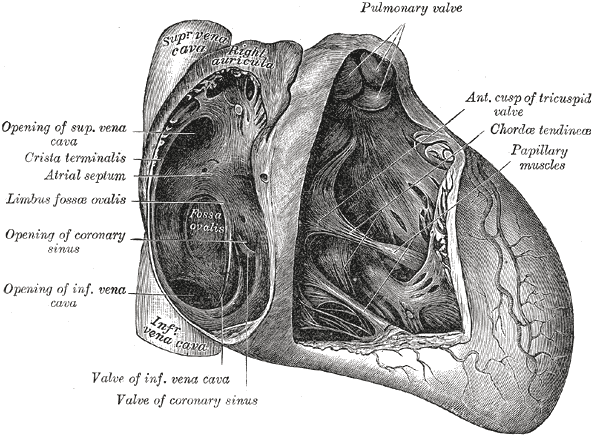
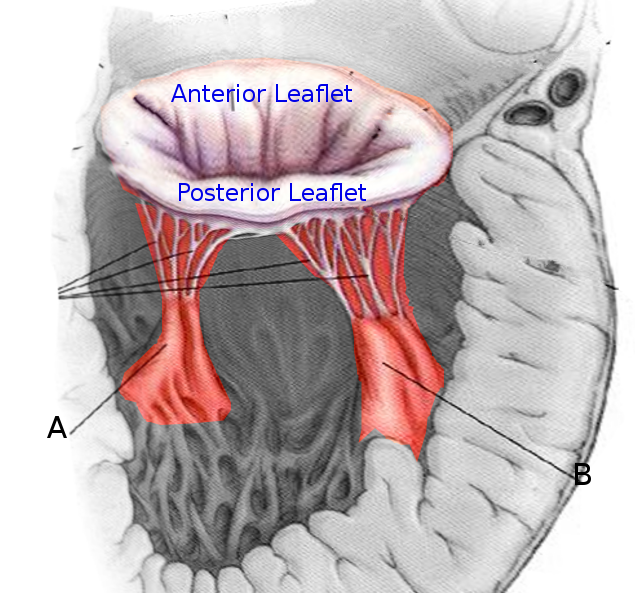
The heart contains 5 papillary muscles originating from the ventricular walls (see Image. Anatomy of the Heart). These muscles attach to the tricuspid and mitral valve leaflets through the chordae tendineae, preventing ventricular blood regurgitation by stabilizing the valves during systole. Three papillary muscles—anterior, posterior, and septal—attach to the tricuspid valve, whereas two—anterolateral and posteromedial—connect to the mitral valve (see Image. Mitral Valve Leaflets). Rupture of the tricuspid papillary muscles can occur due to myocardial ischemia, trauma, or infective endocarditis.[5] Papillary muscle dysfunction causes blood to regurgitate through the valves, leading to backflow of blood that can result in left- or right-sided heart failure.
Papillary muscle rupture was described in the literature as early as 1948. Visualization of rupture through two-dimensional echocardiography was first reported in 1981. Transesophageal echocardiography was first used to identify the condition in 1985.[6][7][8]
Papillary muscle rupture is a rare but severe mechanical complication that can occur following an acute myocardial infarction, affecting 0.07% to 0.26% of patients. Despite the condition's rarity, papillary muscle rupture contributes to 5% of postmyocardial infarction mortality.[9] Papillary muscle rupture leads to severe mitral valve regurgitation, often resulting in cardiogenic shock and pulmonary edema, necessitating immediate medical intervention.
A classic scenario involves a patient with a myocardial infarction affecting the posterior descending coronary artery's territory who develops sudden, decompensated heart failure 2 to 7 days after the infarction. The anterolateral and posteromedial papillary muscles play a key role in maintaining mitral valve function, with the anterolateral muscle receiving a dual blood supply and the posteromedial muscle supplied solely by the posterior descending coronary artery. Due to this single blood supply, the posteromedial papillary muscle is more likely to rupture following a myocardial infarction. Mortality is very high without timely surgical treatment.
Etiology
Papillary muscle rupture most commonly results from myocardial infarction, typically occurring 2 to 7 days after the ischemic event. Rupture occurs more frequently with ST-segment elevation myocardial infarction compared to non–ST-segment elevation infarction. Other documented causes of papillary muscle rupture include trauma, syphilis, periarteritis nodosa, vegetative valvulitis, myocardial abscess, iatrogenic injury, and cocaine use.
Severe acute mitral regurgitation resulting from papillary muscle rupture following acute myocardial infarction is a life-threatening condition if not treated promptly. Although randomized trials are lacking, multiple retrospective studies have consistently shown significantly lower in-hospital mortality rates with timely surgical intervention compared to medical therapy alone.[10] In recent years, single-center studies and national registries have provided comprehensive data on post-acute myocardial infarction papillary muscle rupture, indicating a mean age and male predominance consistent with previous reports. Hypertension, present in over 60% of patients, was the most common cardiovascular risk factor.[11]
Epidemiology
Papillary muscle rupture is a rare complication, estimated to occur in 1% to 5% of patients with acute myocardial infarction. This low incidence is believed to be due to improvements in early identification and early revascularization through percutaneous coronary intervention to limit ischemia. Rupture carries a high mortality rate without surgical intervention, with an estimated 50% mortality within 24 hours in cases of complete rupture. Mortality rates were estimated at 80% to 90% within the first 24 hours before cardiac surgery became a treatment option. A study found that 82% of papillary muscle rupture cases following infarction occurred in patients with their first myocardial infarction.[12][13]
Risk factors for papillary muscle rupture include advanced age, female sex, a history of heart failure, delayed treatment after myocardial infarction, and chronic kidney disease. The mortality rate for papillary muscle rupture can reach up to 75% within the first 24 hours when treated with medical therapy alone and without surgical intervention.
Pathophysiology
Papillary muscle rupture is either partial or complete. A partial rupture, occurring at one of the muscle heads, causes fewer leaflets to flail and results in less valvular regurgitation. Partial ruptures are better tolerated hemodynamically compared to complete ones, which may occur up to 3 months after infarction. Complete rupture of the papillary trunk, typically within a week post-infarction, causes rapid clinical deterioration.
The posteromedial papillary muscle is most often involved due to its single blood supply from the posterior descending artery, the impairment of which can produce inferior wall ischemia. This artery typically originates from the right coronary artery but may also arise from the left circumflex artery. This single blood supply makes rupture of the posteromedial muscle 6 to 12 times more frequent compared to that of the anterolateral muscle, which has a dual blood supply. About 50% of patients have single-vessel disease. Most documented papillary muscle rupture cases demonstrate small areas of ischemia, typically less than 25% of the ventricle, with poor collaterals. In small infarcts, papillary muscle rupture is believed to occur due to preserved ventricular function, which creates high shear forces on the ischemic papillary muscle.
History and Physical
Papillary muscle rupture should be suspected in patients experiencing sudden acute heart failure symptoms within the first week after myocardial infarction, particularly when the inferior wall is involved. Rapid and severe regurgitation from papillary muscle failure leads to atrial dilation due to an abrupt increase in atrial pressure. The combination of a hyperactive precordium and insufficient turbulence of blood flow through the regurgitant valve complicates the clinical diagnosis, often resulting in the absence of an audible regurgitant murmur. This phenomenon occurs because of pressure equalization between the atria and the ventricle, although some patients may exhibit midsystolic, late systolic, or holosystolic murmurs.
Symptoms and physical findings depend on the affected valve, with the posterior-medial papillary muscle of the mitral valve being the most commonly involved. Consequently, symptoms of acute left-sided heart failure, such as rapidly progressive pulmonary edema and hypoxia, are prevalent. Cardiogenic shock, characterized by hypotension, is frequently observed, and some patients may also report chest pain. The rapid onset of cardiogenic shock and the potential for catastrophic complications make prompt identification crucial, as mortality rates increase significantly without emergency surgical intervention.[14]
Evaluation
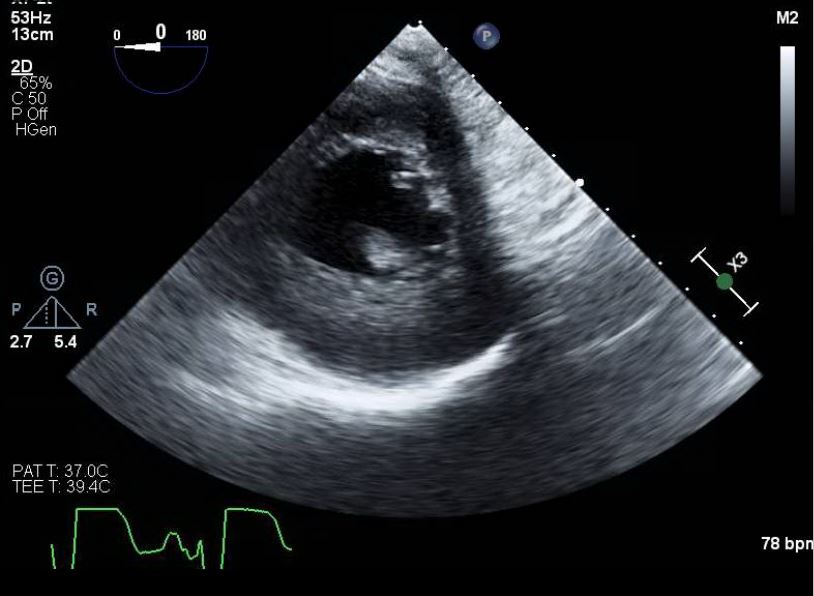
Papillary muscle rupture and acute valvular insufficiency can be identified using transthoracic echocardiography or transesophageal echocardiography. Transthoracic echocardiography may reveal a flail mitral valve leaflet prolapsing into the atrium during systole, a ruptured papillary muscle head exhibiting erratic movements in the ventricle, or a mobile mass attached to the chordae tendineae. The sensitivity of transthoracic echocardiography for visualizing structural abnormalities ranges from 65% to 85%. Transthoracic echocardiography is recommended when transthoracic echocardiography results are inconclusive, as its sensitivity can reach 92% to 100% (see Image. Papillary Muscle Rupture on Transesophageal Echocardiography).[15]
Many patients are too hemodynamically unstable to undergo invasive procedures. Thus, transthoracic echocardiography is typically the initial diagnostic method used. Doppler echocardiography and color flow imaging also help determine the severity of the regurgitant jet across the valve. Echocardiography is superior to cardiac catheterization as muscle ruptures may be diagnosed with minimal risk to the patient. Besides acute valvular surgery, coronary revascularization is strongly recommended, as patients undergoing this treatment have demonstrated improved mortality rates.
Treatment / Management
Patients require careful medical optimization in the early stages of acute mitral regurgitation. Typical measures include:
- Hemodynamic stabilization using vasoactive medications and mechanical circulatory support
- Afterload reduction through the use of an intra-aortic balloon pump
- Management of acute pulmonary edema with intravenous diuretics and ventilatory support
- Treatment of arrhythmias with pharmacologic agents or mechanical cardioversion
- Potential percutaneous intervention for the infarct-related artery
- Monitoring for other possible mechanical complications of myocardial infarction
Once diagnosed, prompt correction of acute mitral regurgitation due to papillary muscle rupture is essential. The treatment approach depends on the patient's comorbidities, the location and extent of the rupture (partial or complete), the anatomy of coronary artery disease, available interventions, and inputs from an interprofessional cardiac team. Treatment options for mitral regurgitation include percutaneous and surgical techniques, though the decision to revascularize during mitral valve intervention may vary by practice.
Surgical intervention remains the gold standard for managing papillary muscle rupture. A treatment controversy exists regarding whether mitral valve repair is more beneficial than valve replacement. Currently, repair is preferred over replacement unless necrotic papillary muscle tissue is present. In addition, concomitant coronary artery bypass has been shown to improve outcomes and should be performed.
Suboptimal outcomes following valve repair have been linked to prolonged cross-clamp times, suturing into friable necrotic tissue, and tissue remodeling after infarction. Surgeons may delay the repair of partial papillary muscle rupture for up to 6 to 8 weeks post-infarction to allow tissue necrosis to resolve. This decision depends on the patient's stability; surgical correction may be required sooner.
Differential Diagnosis
Other complications of myocardial infarction that may present similarly to papillary muscle rupture include cardiogenic shock due to severe left and right ventricular dysfunction, ventricular septal rupture, and free-wall myocardial rupture. These complications are characterized by severe left ventricular dysfunction, which manifests as pulmonary edema and decreased cardiac output, ultimately resulting in organ hypoperfusion. Severe right ventricular dysfunction presents with elevated jugular venous pressure, peripheral edema, hypotension, and clear lung fields. Right ventricular failure may also result in underfilling the left heart chambers, resulting in a low cardiac output state. Ventricular septal rupture has a 5% mortality rate and may be associated with anterior infarction, whereas papillary muscle rupture is infrequently observed in anterior cardiac ischemia.
Septal rupture occurs where necrotic tissue is located, creating a left-to-right shunt and a new pansystolic murmur. Rapid pulmonary edema is typically not observed. Free-wall myocardial rupture, similar to septal and papillary muscle rupture, is often caused by small infarctions and single-vessel disease. The left ventricular wall is the most common rupture site and occurs within 5 days in 50% of patients and 2 weeks in 90% of patients. Survival depends on whether the rupture is complete or subacute, with complete ruptures having a nearly 100% fatality rate due to the abrupt onset of cardiac tamponade.
Prognosis
A study involving 22 patients reported a perioperative mortality rate of 27% and overall survival of 47% at 7 years. Another study involving 55 patients indicated an overall operative mortality rate of 24%. The mortality rate was notably higher in patients who did not undergo coronary revascularization, with a rate of 39% compared to 9% in individuals who received the procedure. In addition, in a study involving 54 patients, the 10-year survival rate was reported to be 35%, and the 10-year heart failure-free rate was 23%.
Several adjusted risk factors have been linked to increased inpatient mortality in patients with ST-elevation myocardial infarction-related papillary muscle or chordae tendineae rupture. For every 5-year increase in age, the risk of in-hospital mortality rose by 9% (aOR: 1.09, 95% CI: 1.07–1.12, p < .001). Female sex (aOR: 1.87, 95% CI: 1.44–2.41, p < .001), Hispanic ethnicity (aOR: 7.05, 95% CI: 3.39–14.64, p < .001), and coagulopathy (aOR: 1.48, 95% CI: 1.06–2.07) were all associated with higher mortality. In addition, comorbid conditions, such as hypertension, smoking, cardiac arrest, and cardiogenic shock, further contributed to an increased risk of in-hospital mortality.
Complications
The early and more assertive use of short-term mechanical circulatory support devices has shown positive outcomes in patients experiencing postcardiotomy cardiogenic shock. Understanding the specific hemodynamic effects of each mechanical circulatory support device is crucial for selecting the most appropriate therapy, particularly in acute severe mitral regurgitation cases. Simulations have provided valuable insights into the hemodynamics of these devices. Although extracorporeal membrane oxygenation is commonly employed for patients with postcardiotomy shock, simulations suggest that this modality may exacerbate severe mitral regurgitation, necessitating additional measures to unload the left ventricle.
Consultations
Immediate consultation with cardiology and cardiothoracic surgery is indicated in cases of suspected papillary muscle or chordae tendineae rupture. Timely intervention is crucial to prevent severe complications and improve patient outcomes.
Pearls and Other Issues
Acute papillary muscle or chordae rupture and secondary acute valvular regurgitation should be considered in any patient presenting with acute pulmonary edema or shock, especially in the days following an acute myocardial infarction. These patients are highly hemodynamically unstable, necessitating urgent consultation with cardiology and cardiovascular surgery. Mortality may be substantially reduced with rapid identification and referral for valvular repair or replacement and revascularization. The best initial study for identification in an unstable patient is transthoracic echocardiography, whereas transesophageal echocardiography is reserved for individuals with equivocal transthoracic echocardiography studies.
Enhancing Healthcare Team Outcomes
The increasing recognition of the complexities of modern cardiovascular care has prompted a reassessment of management strategies and staffing requirements within today's cardiac intensive care unit. Multiorgan system involvement is now frequently encountered and often necessitates input from multiple disciplines to achieve optimal outcomes. Collaborative decision-making, led by a clinician with critical care expertise, has improved teamwork and communication, streamlined care transitions, and even boosted patient survival rates. Patient-focused, team-based care can enhance adherence to best practice guidelines, minimize adverse events, lower healthcare costs, and increase patient and family satisfaction.
Ideally, myocardial infarction is managed by an interprofessional team that includes cardiology nurses. The patient must be prepared for immediate surgery when papillary muscle rupture is diagnosed. One challenge is deciding whether to perform cardiac catheterization on an unstable patient. Some surgeons proceed to the operating room without it, assuming the involvement of the right coronary or posterior descending artery. Due to the extremely high mortality of papillary muscle rupture, the focus today is on the prevention of coronary artery disease. The cardiology nurse, pharmacist, and primary clinicians should educate the patients on the following:
- Engaging in regular exercise
- Staying on a healthy diet
- Complying with prescribed medication regimens
- Abstaining from alcohol drinking and cigarette smoking
- Taking statins as prescribed
- Maintaining a healthy body weight
- Monitoring and maintaining normal blood pressure and blood glucose
Patients with risks for coronary artery disease must be closely followed and referred to a cardiologist for definitive care. Close communication between the team members is vital to improve outcomes. The prognosis for these patients is guarded. The disorder is fatal without surgery, and recovery is slow even with surgery.