Continuing Education Activity
Osteonecrosis is a degenerative bone condition characterized by the death of cellular components of the bone secondary to an interruption of the subchondral blood supply. Also known as avascular necrosis, it typically affects the epiphysis of long bones at weight-bearing joints. The most common sites for AVN are the femoral head, knee, talus, and humeral head. The hip is the most common location overall. Advanced disease may result in subchondral collapse, which threatens the viability of the joint involved. Therefore, early recognition and treatment of osteonecrosis are essential. This activity discusses the etiology and pathogenesis of the disease, presentation, and treatment options of the most common forms of osteonecrosis.
Objectives:
Identify the etiology of osteonecrosis.
Review the presentation of a patient with osteonecrosis.
Outline the treatment and management options available for osteonecrosis.
Summarize interprofessional team strategies for improving care and outcomes in patients with osteonecrosis.
Introduction
Osteonecrosis is a degenerative bone condition characterized by the death of cellular components of the bone secondary to an interruption of the subchondral blood supply.[1] It is also known as avascular necrosis, aseptic necrosis, and ischemic bone necrosis. It typically affects the epiphysis of long bones at weight-bearing joints. Severe cases can lead to the destruction of subchondral bone or the collapse of an entire joint.[1]
The most common sites for AVN are the femoral head, knee, talus, and humeral head. The hip is the most common location overall.[2] AVN less commonly occurs in other bones of the body, such as the carpus and jaw.[3][4] Therefore, early recognition and treatment of osteonecrosis are essential.[2] This activity discusses the etiology and pathogenesis of the disease. Additionally, it includes the presentation and treatment options of the most common forms of osteonecrosis.
Etiology
The widely accepted view in the literature is that a reduction in subchondral blood supply is responsible for osteonecrosis. However, numerous risk factors and theories exist on the development of this vascular impairment. Shah et al. succinctly categorizes these into six groups.[5] These are:
- Direct cellular toxicity
- Chemotherapy
- Radiotherapy
- Thermal injury
- Smoking
- Extraosseous arterial fracture
- Hip dislocation
- Femoral neck fracture
- Iatrogenic post-surgery
- Congenital arterial abnormalities
- Extraosseous venous
- Venous abnormalities
- Venous stasis
- Intraosseous extravascular compression
- Hemorrhage
- Elevated bone marrow pressure
- Fatty infiltration of bone marrow due to prolonged high-dose corticosteroid use
- Cellular hypertrophy and marrow infiltration (Gaucher disease)
- Bone marrow edema
- Displaced fractures
- Intraosseous intravascular occlusion
- Coagulation disorders such as thrombophilias and hypofibrinolysis
- Sickle cell crises
- Multifactorial
In a small percentage of cases, mutations in the COL2A1 gene, which codes for type 2 collagen production, have demonstrated autosomal dominant inheritance patterns.[6] However, in many cases, a cause cannot be identified, and these patients receive the designation of idiopathic osteonecrosis.
Repetitive trauma, like assembly line work, can lead to AVN over time. Nontraumatic risks for AVN include anything potentially impacting vascular flow to the bone. Radiation can induce bone marrow changes, leading to AVN.[7] Hyperlipidemia causes significant blockage of small blood vessels, reducing the blood flow that provides bone with nutrients. Medical conditions like sickle cell anemia can also diminish the vascular supply to the bone.[8]
Anatomy with low collateral capabilities or retrograde vasculature, such as the carpus, is at increased risk for necrosis.[9] Unique anatomy also plays a role in the talus, where a considerable portion of its surface area consists of articular cartilage, which also restricts opportunities for blood flow.[10]
Glucocorticoids used at high doses and for prolonged periods can induce osteonecrosis via osteocyte apoptosis. Apoptosis disrupts the lacunar-canicular system.[2] Other risk factors for AVN are alcohol misuse, blood dyscrasias, autoimmune diseases such as lupus, etc. The cause of interrupted blood flow is unknown for twenty-five percent of people with AVN. For example, in Kienböck and Preiser disease, the exact cause of AVN is usually inexplicable.[3][11]
Epidemiology
Osteonecrosis is most common in the hip but is also seen in the humerus, knee, and talus and is rarely seen in the smaller bones of the wrist, such as the lunate. While the jaw can be affected, this review focuses on more common forms presented to an orthopedic surgeon.
Ten percent of total hip arthroplasties in the United States are due to AVN and typically affect ages 30 to 65. Males tend to be more affected by ON overall, but autoimmune conditions affecting women, like lupus, are also significant.[1] Less common variants, such as Preiser disease (osteonecrosis of the scaphoid), tend to affect the dominant hand of middle-aged men most.[12] In contrast, Kienböck disease (osteonecrosis of the lunate) is more common in middle-aged males involved in manual labor, and there have even been case reports involving children.[13]
Pathophysiology
A reduction in subchondral blood supply induces a state of hypoxia, leading to loss of integrity of cell membranes and necrosis of cells. The pathological appearances of necrosis marked by the appearance of neutrophils and macrophages will predominate. Macroscopically, this induces subchondral collapse and subsequent joint degeneration.
MRI will demonstrate osteosclerotic changes secondary to decreased bone resorption as a result of disrupted osteoclast function. Specifically, the T2 signal will be increased, and the T1 signal will be decreased due to fat cell edema/ischemia of bone marrow.[9]
Histopathology
Osteocytes undergo apoptosis, and phagocytosis cannot occur. Thus, the osteocytes are not replaced. This process leads to poor bone remodeling and osteosclerosis.[9]
History and Physical
Non-traumatic cases will typically present with mechanical pain of variable onset and severity and are often difficult to localize. In early disease, the physical examination is usually normal and inevitably causes a delay in diagnosis.[4] A focused history should include recent trauma, steroid use, autoimmune disease, sickle cell, alcoholism, tobacco use, manual labor, change in gait, connective tissue disorders, insidious onset pain, and decreased range of motion.[8]
Osteonecrosis of the hip often has early stages that are often asymptomatic. Hip and groin pain are the most common presenting symptoms and usually indicate late-stage progression. Associated symptoms can include referred pain in the buttock and thigh. The majority of patients have pain at rest. Others include stiffness and changes in gait.[1]
Osteonecrosis of the knee most commonly presents as acute onset knee pain that occurs while weight-bearing and at night. A history of osteoporosis or osteopenia and no recent trauma are typical responses. On physical exam, pain with palpation over the medial femoral condyle and decreased range of motion are observed.[14]
Osteonecrosis of the shoulder, involving the proximal humerus, is often associated with trauma and osteonecrosis elsewhere in the body. Pain is characterized as pulsating with radiation to the elbow and a decreased active range of motion.[15]
Osteonecrosis of the talus is associated with polyarticular disease and trauma. Patients often complain of pain and difficulty ambulating well beyond the expected recovery time following a traumatic event.[16][10]
Osteonecrosis of the lunate and scaphoid most often presents without a history of trauma. Patients are usually skilled laborers and complain of unilateral pain in the wrist's dorsal and radial aspects. Other common findings include decreased range of motion, wrist swelling, and weakened grip strength.[13][17]
Evaluation
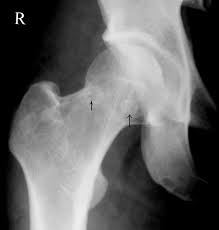
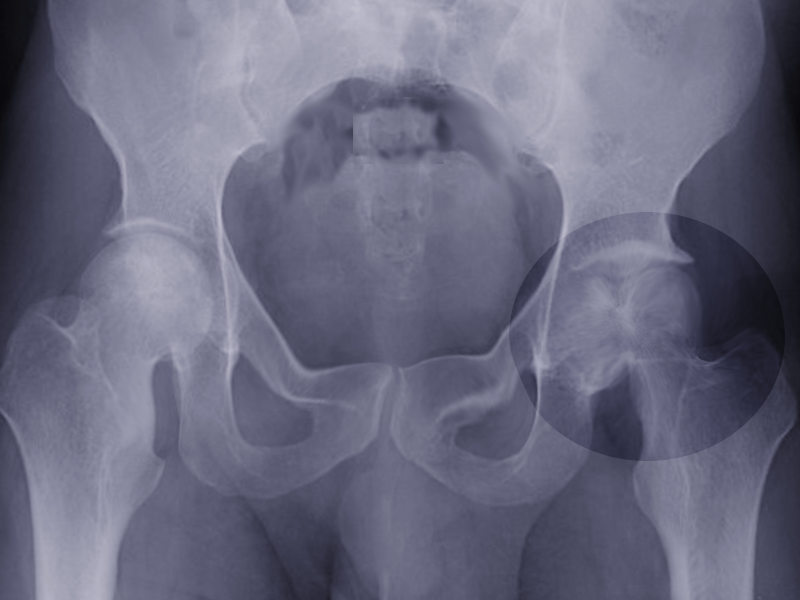
Initial evaluation with plain radiographic films demonstrating two orthogonal views is the initial diagnostic standard, although they often appear normal in the early course of the disease. Magnetic resonance imaging is recommended for detecting earlier stages of the disease due to its high sensitivity in detecting bone edema.[5]
Classification
Osteonecrosis of the Hip
Femoral head osteonecrosis falls into two classes: traumatic and atraumatic. Of the atraumatic cases, up to 70% may be bilateral.[18] Common classifications that map the phases of osteonecrosis of the hip include the Ficat and, Arlet, and Steinberg classifications.[19][20] Ficat and Arlet describe the four stages of disease progression based on clinical and radiographic findings. Stage 1 is the initiation of the disease without radiological findings. Stage IV is the end stage with femoral head collapse, flattening, and narrowing of the joint space. The Steinberg classification incorporates the use of MRI to detect a pre-clinical lesion and also to assess the size of the lesion.
Osteonecrosis of the Knee
Spontaneous osteonecrosis of the knee (SONK) is the most common type.[21] Secondary osteonecrosis is commoner in a younger population and is often associated with several risk factors common to all kinds of osteonecrosis, as previously discussed. The third and rarest type is post-arthroscopic osteonecrosis, which has been seen in 4% of patients having undergone an arthroscopic meniscectomy. SONK typically presents in the sixth decade of life and is more common in the female population. Classically, it affects the medial femoral condyle (up to 94% of cases), and subsequent cadaveric studies have demonstrated a watershed area of the medial femoral condyle.[22][14] The overriding symptom is medial knee pain, often mimicking a torn meniscus. The Koshino classification describes the four stages of disease progression.[22] Stage 1 describes clinical disease without radiologically apparent disease. Stage 4 is the degenerative phase with osteosclerosis and osteophyte formation around the condyles.
Osteonecrosis of the Shoulder
Osteonecrosis of the shoulder most frequently results from trauma; however, it can arise from the causes outlined above, for instance, prolonged high-dose corticosteroid usage. Hertel et al.[23] showed that specific fracture plane combinations had associations with impaired head perfusion and that additional elements, such as the length of the posteromedial metaphyseal head extension and the integrity of the medial hinge, were the key elements for the occurrence of osteonecrosis. Most fracture patterns with an increased risk of avascular necrosis had an anatomic neck component. Interestingly, fracture-dislocations and degree of displacement of the fragments do not predict an increased incidence of avascular necrosis of the humeral head, although contradictory evidence does exist. Classification is the Cruess classification and uses five categories.[24] Stage 1 describes normal X-ray findings with pathology only recognized on MRI scans. Stage four demonstrates flattening of the humeral head with collapse. Stage five is the end stage, with degenerative changes that extend to the glenoid.
Osteonecrosis of the Talus
Most commonly caused by trauma resulting in displaced fractures to the neck of the talus. The incidence of avascular necrosis increases with co-existing dislocation at the ankle or subtalar joint. The talus body is often affected by a predominate cartilaginous surface, limiting its vascular supply.[25] The Hawkins classification best describes the disease progression. If osteonecrosis is to occur, the pathognomonic subchondral lucency known as the Hawkins sign will be absent on plain radiographs at 6 to 8 weeks.
Osteonecrosis of the Lunate
More commonly known as Keinbock disease involves a collapse of the lunate due to vascular insufficiency and osteonecrosis. A history of repetitive trauma, biomechanical factors related to ulna variance, and anatomic factors such as the presence of both dorsal and palmar blood supply may contribute to the risk of avascular necrosis. The Lichtman staging of Keinbock disease uses four stages. The first radiographic feature becomes apparent in stage 2 disease, represented by lunate sclerosis without height loss.[17] Lunate collapse and palmar scaphoid rotation are the radiologic features in stage 3 disease. Intercarpal joint degeneration is the dominant feature of end-stage disease.[17]
Osteonecrosis of the Scaphoid
Known as Preiser disease, it arises from an uncertain etiology. Risk factors include alcohol abuse, trauma, steroid use, and connective tissue disorders.[3][12] Typically, it affects middle-aged males' dominant hand, although it can present bilaterally. A radiograph shows scaphoid sclerosis early in the disease process. Late-stage findings demonstrate cystic changes and fragmentation. MRI may detect T1/T2 signal changes throughout the scaphoid or specific to the proximal pole. The Herbert and Lanzetta classification system is used to assess progression. The system consists of four stages that begin with changes to the scaphoid's proximal pole, followed by eventual carpal collapse.[12]
Treatment / Management
Osteonecrosis of the Hip
Nonoperative treatment ( Alendronate only therapy and bisphosphonates combination) is controversial, with some trials showing the benefit of bisphosphonates in preventing a femoral head collapse and delaying disease progression in early cases with subchondral lucency. However, its delayed onset of pain relief can compromise its compliance.[26]
Many patients will ultimately need a total hip arthroplasty; however, joint salvaging procedures such as core decompression report varying results. Core decompression is most effective in the early stages of osteonecrosis and when the lesions only involve a small amount of the weight-bearing surface of the femoral head.[27] The procedure may use vascularised bone grafts or biological agents that promote bone repair. Core decompression is safe and effective in the early stages of osteonecrosis. Its use, combined with using autologous bone or bone marrow, can increase its success rate.[28]Interestingly Zhao et al. used perfusion studies to show core decompression is more effective in femoral heads with venous congestion than those with arterial compromise.[29] Core decompression is indicated when etiology is reversible to reduce intraosseous pressure and stimulate bone healing. Traditionally, it was performed using an 8 or 10-mm drill through the subchondral sclerotic area. Alternatively, a 3.2 mm guide wire can be drilled multiple times to decompress the lesion.
Rotational osteotomy can be indicated when the lesion can be diverted from the weight-bearing surface. Medial diseases can be rotated through intertrochanteric varus osteotomy, while the anterolateral disease can be rotated via intertrochanteric valgus and flexion-producing osteotomy. [30] Rotational osteotomy has a success rate of up to 90%; however, it can make future total hip arthroplasty more challenging.[31][32]
Total hip arthroplasty is indicated for irreversible etiologies and when patients are older than 40 years and have large lesions or in young with more advanced femoral head collapse and degenerate acetabulum. Cementless prostheses for both the femur and acetabulum are predominantly used; however, extreme caution must be taken during femoral canal preparation to avoid perforation. Outcomes are usually good in terms of pain reduction and functional improvement. However, a high rate of polyethylene wear and osteolysis have been reported in young patients with osteonecrosis.[33][34]
Hip arthrodesis would be indicated only in young patients involved in heavy labor occupations.
SONK (Spontaneous Osteonecrosis of the Knee)
Most cases resolve following a trial of protected weight-bearing and physiotherapy focused on quadriceps strengthening. In recent literature, bisphosphonates have been shown to reduce the need for surgery by 50% by delaying postnecrotic remodeling and preventing joint surface collapse.[35] SONK is more common in older persons, so a unicompartmental knee replacement provides a good functional outcome with a relatively short rehabilitation time.[21] However, a total knee replacement may be more appropriate in larger lesions. Smaller lesions following intraosseous decompression have achieved good operative results. High tibial osteotomy will have a role in cases associated with malalignment. Joint-preserving procedures such as a combination of arthroscopy and core decompression or osteochondral autograft transfer have successfully postponed knee arthroplasty in selected patients SONK in the precollapse stage.[36]
Osteonecrosis of the Shoulder
Nonoperative treatment is the first line of treatment: pain control, physiotherapy, and modification of activities to restrict overhead activities and manual labor.
Operative management is classified according to staging. For early disease, core decompression is the preferred treatment option. Humeral head resurfacing or hemiarthroplasty is recommended for moderate disease with focal cartilage defects and sufficient epiphyseal bone stock. A total shoulder replacement is reserved for advanced disease.[10]
Osteonecrosis of the Talus
The incidence of osteonecrosis of the talus in talar neck fractures is reduced by utilizing a procedure to achieve operative anatomic reduction and stable fixation.[5]
Keinböck Disease
Treatment in early-stage disease aims to revascularize the lunate either directly using bone grafts or indirectly utilizing procedures to offload the lunate. Immobilization, including external fixation, is often attempted in stage 1 and 2 diseases.[17] Surgical options address the carpal collapse in stage 3, whereas advanced disease may warrant joint sacrificing procedures such as wrist arthrodesis.[17]
Preiser Disease
Early-stage treatment options involve immobilization, cortisone injections, radial wedge osteotomy, and bone graft. Later stages may warrant arthroscopic debridement, scaphoid excision, proximal row carpectomy, or even arthrodesis. Typically, surgical intervention is unavoidable in most cases.[12]
Differential Diagnosis
- Bone marrow edema syndrome (aka, transient osteopenia)[1]
- Complex regional pain syndrome
- Inflammatory synovitis
- Neoplastic bone conditions
- Osteoarthritis
- Osteochondrosis[9]
- Osteoarthritis
- Osteomyelitis
- Osteoporosis
- Rheumatoid arthritis
- Septic arthritis
- Soft tissue trauma (eg, labral tears, meniscal tears)[14]
- Subchondral fractures[1]
Prognosis
The prognosis for osteonecrosis is often poor, regardless of the initial management strategy. Progression of the disease includes persistent pain, debilitation, and destruction of the joint beyond repair. Mont et al.reported that 59% of asymptomatic lesions progressed to symptoms or collapse.[2] AVN of the humeral head, in particular, can be even worse, with as many as 81% of patients progressing to total failure and requiring arthroplasty.[8] Therefore once the patient faces the onset of osteonecrosis, there is a high probability that it will continue to advance.
Core decompression and bone grafting can prevent the progression of osteonecrosis of the hip. However, with advanced disease or failure of joint-preserving therapies, total hip arthroplasty is needed but is associated with increased complication rates.[37]
Core decompression and hemiarthroplasty of the shoulder have good outcomes in treating AVN in the early stages. However, total shoulder arthroplasty has higher complication rates. Total shoulder arthroplasties are indicated for end-stage AVN but put the patient at risk for postoperative complications.[15]
In Preiser and Kienböck disease, the initial stages of AVN are treated with immobilization, but eventual surgical intervention is usually indicated.[12][17] Surgical repair of the talus in AVN management tends to have significantly better outcomes in younger patients.[10]
Complications
Common postoperative complications for osteonecrosis include surgical site infections, prosthesis malfunctions, and neurovascular compromise.[15] High failure rates occur as the disease continues to progress despite surgical intervention. For example, core decompression failed to prevent disease progression in 90% of patients treated for AVN of the hip.[8] In Kienböck disease, not taking into account the patient's unique anatomy when performing an osteotomy of the radius can result in excessive positive ulnar variance and ulnar pain postoperatively.[17]
Patient comorbidities greatly influence complication rates as well. For example, patients with sickle cell disease undergoing total joint replacement for AVN had more extended hospital stays, increased likelihood of acute kidney injury, implant failure, pulmonary embolism, deep vein thrombosis, myocardial infarction, and an overall higher mortality rate.[8]
Deterrence and Patient Education
Osteonecrosis often starts even before a patient experiences symptoms. It typically progresses to end-stage disease if left untreated. Symptoms vary, but patients with late-stage disease usually have severe pain, debilitation, and even joint collapse.[1]
Patient presentation of this disease is highly variable, so it is essential to reduce your risk factors. Ways to decrease risks include avoiding excessive alcohol consumption, no tobacco use, and tapering corticosteroids to the lowest necessary dose. Other lifestyle modifications like eating a healthy diet and maintaining an appropriate weight minimize joint damage. For manual laborers, consider avoiding repetitive activities that place strain on their joints. If possible, laborers should take regular breaks. Lastly, be proactive. Since patients with early-stage often do not have symptoms, they should seek periodic well-being evaluations by their primary care clinician.[1]
Pearls and Other Issues
There are several identified risk factors for osteonecrosis, but the exact pathogenesis remains unestablished. More likely, a combination of different factors and conditions leads to the destruction of bone cells.
Intervention depends on the phase of disease progression and varies from preservation procedures to more definitive salvage operations.
Treatment of early-stage disease reports better results, although this may be difficult to achieve as osteonecrosis is often asymptomatic in its early stages.
The majority of non-traumatic cases are associated with glucocorticoid use.[2]
Enhancing Healthcare Team Outcomes
Osteonecrosis frequently poses a diagnostic dilemma to orthopedic surgeons. Several risk factors are outlined in the literature, yet the exact pathophysiology is not fully understood. Diagnostic difficulty frequently results in delayed presentation of advanced cases of the disease, which often prevents the joint from being salvaged and increases patient morbidity. While an orthopedic surgeon is almost always involved in caring for patients with osteonecrosis, educating healthcare professionals who encounter these patients at the hospital front door, such as general practitioners, emergency department physicians, nurses, and physiotherapists, is essential. This way, a well-functioning interprofessional healthcare team approach to patient care in these cases is possible, with each member contributing from their area of expertise, exercising open communication with the rest of the team, and documenting all interactions with the patient so that the appropriate caregiver can be contacted if changes in the patient's regimen or rehabilitation are necessary. This interprofessional approach will yield the best patient results. [Level 5]