Continuing Education Activity
Osteochondromas are common benign osseous surface lesions, generally arising from the metaphysis of long bones; they are most commonly asymptomatic and found incidentally. However, there are several well-documented complications, including but not limited to fracture, bursa formation, neurovascular compression, and malignant degeneration. This activity reviews the pathophysiology and etiology of osteochondromas and highlights the role of the interprofessional team in evaluation and management.
Objectives:
Determine the etiology of osteochondromas.
Assess the differences in solitary (nonhereditary) and various hereditary multiple exostoses forms.
Identify the clinical presentation of osteochondromas.
Collaborate with the interprofessional team in the diagnosis and management of osteochondrosis to help improve outcomes.
Introduction
Osteochondromas represent the most common bone tumor, accounting for 20% to 50% of all benign osseous tumors.[1][2] Osteochondromas are surface bone lesions composed of both cortical and medullary bone with hyaline cartilage caps. The presence of cortical and medullary continuity of the tumor with the underlying bone is a pathognomonic feature that establishes the diagnosis.[1][2][3] Osteochondromas may be solitary or multiple. The multiple form is an autosomal dominant syndrome referred to as hereditary multiple exostosis (HME) or familial osteochondromatosis.
Osteochondromas can be an incidental finding or present with a palpable mass or symptoms due to compression of the adjacent structures, which varies based on the involved structure.[2][4][5][6][7][8][9][10] Complications associated with osteochondromas are common, including osseous deformities, fractures, bursa formation with or without bursitis, vascular compromise, neurologic symptoms, and malignant transformation.[1][2][3][11]
Radiographs are often diagnostic; however, cross-sectional imaging may be indicated to assess for complications, assess the cartilage cap, or, in some challenging cases, establish the presence of medullary continuity. The lesions may increase in size in skeletally immature patients; however, growth or changes in morphology after skeletal maturation are concerning features for malignant transformation. The lesions may be sessile or pedunculated. When pedunculated, they extend away from the nearest joint.[1]
There are multiple osteochondroma variants or mimickers. Including subungual exostosis, dysplasia epiphysealis hemimelica (Trevor disease), turret exostosis, traction exostosis, bizarre parosteal osteochondromatous proliferation (BPOP) (or Nora lesion), and florid reactive periostitis.[1][12] Differential considerations also include subperiosteal hematoma, parosteal osteosarcoma, or juxtacortical chondroma, none of which have medullary continuity.[2][3][12][13][14]
Nonoperative treatment is indicated for the majority of solitary osteochondromas. Retinoid acid receptor gamma (RARy) agonists are an emerging potential treatment.[15][16][17] Operative management can involve marginal surgical excision of the lesion, with wide surgical excision reserved for cases of malignant transformation.[4][18][19][20][21]
Etiology
Osteochondroma can present as a solitary lesion or part of numerous osteochondromas in patients with HME.[1] Solitary lesions can be primary or secondary. Primary osteochondromas develop spontaneously with no precipitating etiology, whereas secondary osteochondromas can result from Salter-Harris fracture, postsurgery, or radiation therapy. Osteochondroma is the most common benign radiation-induced bone tumor, with postirradiation prevalence ranging from 6% to 24%. The latency period for post-radiation osteochondromas ranges from 3 to 17 years.[1][22][23]
Secondary osteochondromas can also be a malignant transformation of a solitary osteochondroma or HME. Secondary osteochondroma is a low-grade tumor that is rare in the pediatric population but is more common in older age groups (50 years and older). The pelvis is the most common location of osteochondroma.
Epidemiology
Osteochondroma is the most common benign bony tumor, accounting for 30% (range 20%-50%) of all benign bony tumors and 10% to 15% of both benign and malignant bony tumors combined. Almost 1% to 2% of patients undergoing radiographic evaluation will have an incidental lesion. Osteochondromas most commonly are present in the first four decades of life, and almost 75% of these lesions are present before the age of 20 years.[4][12] Osteochondromas have a male preponderance.[3][24][25] However, the actual incidence of osteochondroma is unknown, as these tumors are often asymptomatic and undiagnosed; additionally, the incidence varies depending on the type. Solitary osteochondromas are very common (almost 6 times more common than HME) and represent 85% of all the presenting osteochondromas; the remaining 15% present in the form of HME.[18]
The hereditary form (HME) is autosomal dominant with incomplete penetrance in females. Family history is reported in 65% of cases.[26][27][28] In the West, the frequency of HME represents about 1.5% per year, with an incidence of 1 out of 50,000 persons.[24][29][30] HME is usually encountered during the first decade of life (in more than 80% of cases), with a male-to-female ratio of 3:1 and more prevalence in Caucasians.[4][24][25]
The femur is the most commonly affected long bone (30% of osteochondroma cases), with distal lesions more common than proximal ones. The tibia and humerus are the next most common long bones to be affected, each constituting 10% to 20% of cases. Proximal tibia involvement is more common than distal; therefore, osteochondromas about the knee are extremely common. Osteochondromas originating from flat bones (pelvis, scapula, and spine) are rare, and medullary continuity is less evident on the radiographs.[2][22][23] Osteochondromas originating from the vertebrae and ribs are even more rare, with only a few cases reported in the literature.[31] Since the lesions develop from cartilage cells displaced from the growth plates, any bone that develops via enchondral ossification may develop an osteochondroma. The long bones constitute the majority of osteochondroma cases (50%), with the lower extremity to upper extremity ratio being 2:1.
Pathophysiology
Solitary Osteochondroma
Solitary osteochondroma may be a developmental lesion arising from a hamartomatous bone and cartilage proliferation rather than representing a true neoplasm.[1] This hypothesis has support from reported cases of osteochondroma developing following trauma or irradiation.[1] Osteochondroma may also develop from growth plate cartilage fragments that permeate the cortex by endochondral ossification under the periosteum. Growth extension to the surface may be allowed through a defect in the perichondral node of Ranvier. The stalk of the osteochondroma is formed from the ossified cartilage (cortical and cancellous bone).
More recent studies suggest that osteochondromas may be true neoplasms, as genetic mutations have appeared in both HME and solitary forms.[32] Osteochondromas are generally inherited as an autosomal dominant condition. Mutations have been reported in the gene encoding exostosin 1 (EXT1).[1][32] Additionally, loss of regulation in the Indian hedgehog protein was reported to be involved in the pathogenesis of osteochondroma.
HME
The hereditary form of the osteochondroma (HME) is associated with a loss-of-function type of mutation in the tumor suppressor genes EXT1 and EXT2 that are responsible for the synthesis of heparan sulfate proteoglycans (HSPG), which results in HSPG deficiency and subsequent development of multiple osteochondromas.[32] The importance of HSPG in the development of osteochondromas lies in its ability to interact with the bone morphogenetic proteins (BMPs) that have an essential role in the regulation of bone and cartilage formation.[32].
HME follows an autosomal dominant inheritance pattern with incomplete penetrance and a male predominance. A broad spectrum of EXT mutations is associated with EXT1 and EXT2 genes, resulting in HME.[32] Patients with EXT1 mutations are generally more severely affected (more osteochondromas and more severe osseous deformities). Interestingly, even within family members with the same EXT mutations, a variable expression of the HME severity suggests a complex, incompletely understood pathophysiology.[32]
Histopathology
Macroscopic Gross External Examination
Grossly, osteochondroma is a lobulated sessile or pedunculated lesion arising from the bone surface with a somewhat cauliflower-like appearance. The cartilage cap has a shiny, glistening, bluish-grey appearance. There is a thin fibrous capsule or perichondrium that shows continuity with the periosteum of the underlying bone. The cartilaginous cap is usually thin, about 2 to 3 mm. Increased thickness of the cartilaginous cap might indicate further growth of the lesion but does not necessarily confirm malignant degeneration in pediatric patients. In adults, thickening of the cartilaginous cap should be alerting for malignant transformation to chondrosarcoma. Varying degrees of mineralization may be present within the cartilage cap.[23][33]
Macroscopic Cross-sectional Examination
The perichondrium, cortex, and medulla of the osteochondroma are all continuous with the underlying bone.[23]
Microscopic Examination
Osteochondroma has a characteristic histologic appearance similar to a normal growth plate. The cartilaginous cap is formed of primary trabeculae of hyaline cartilage and linear clusters of active chondrocytes with a surrounding well-defined perichondrium. Evidence of endochondral ossification is visible at the junction of the cartilaginous cap and the underlying bone. Through endochondral ossification, the medullary part is usually formed by yellow marrow rather than hematopoietic marrow.[23]
History and Physical
Incidental and Palpable Mass Presentation
The most common presentation of osteochondroma is an incidental finding on radiographs [2][4]. The second most common presentation is a painless, palpable lump on the hosting bone.[3] The palpable lumps can be aesthetically displeasing, especially in the easily observable and palpable areas such as the proximal tibia or the ribs.[34] Other symptoms include mechanical compression on adjacent structures, bursitis, fracture, or malignant transformation.[35]
Neurovascular Symptoms
Patients can present with symptoms of nerve compression, such as tingling and numbness.[5][6] Symptoms due to vascular compression include changes in skin color, loss of pulses, or changes in blood flow. Patients may also develop arterial or venous thrombosis, aneurysm, or pseudoaneurysm. With the knee being the most commonly involved part of the body, the popliteal artery, common peroneal nerve, and posterior tibial nerve are the most frequently involved structures.[7][8][9][10]
Soft Tissue Irritation
Occasionally, an osteochondroma can present inferior to tendons with resultant tendon pain and irritation during movement.[4] Soft tissue compression by the underlying osteochondroma can precipitate bursal formation and bursitis development.[3]
Spinal Symptoms
Osteochondromas adjacent to the intervertebral discs could result in kyphosis or spondylolisthesis.[36] Most osteochondromas develop outside the spinal cord, so it is rarely present with spinal cord compression.[37][38][39]
HME
HME is usually asymptomatic in newborns, whereas almost 50% of the patients would present with a visible tumor diagnosed at age 5 and 80% by age 10.[29] Those patients can present with various valgus deformities such as ankle valgus, genu valgum, and coxa valga. Limb length discrepancies, radial or ulnar deviation, metacarpal or metatarsal or phalangeal shortening. HME patients can also present with acetabular dysplasia, femoroacetabular impingement, or Juvenile arthritis. Presentations such as dislocation or subluxation of the hip, patella, or talus are also common.[4][18][40]
Evaluation
Radiographs
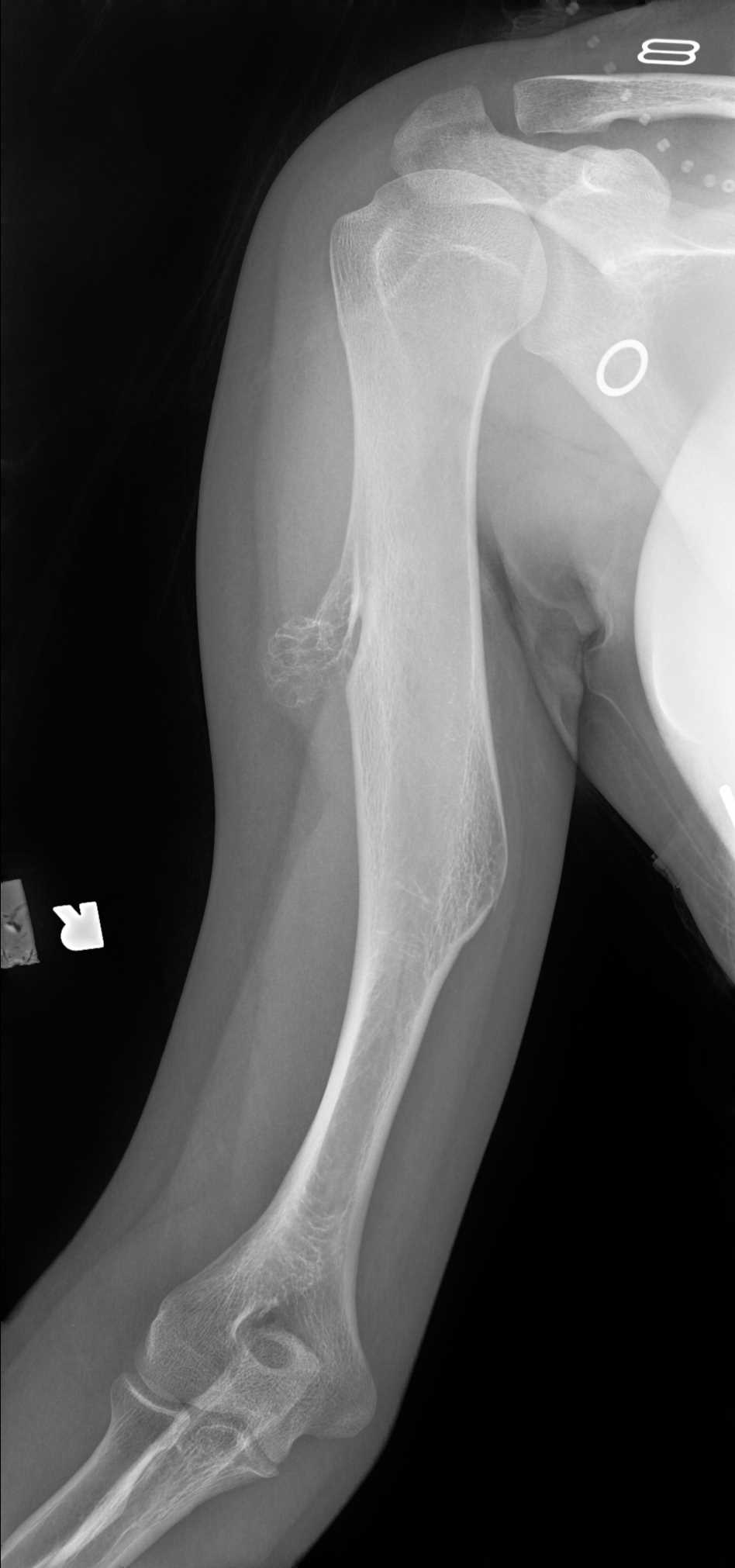
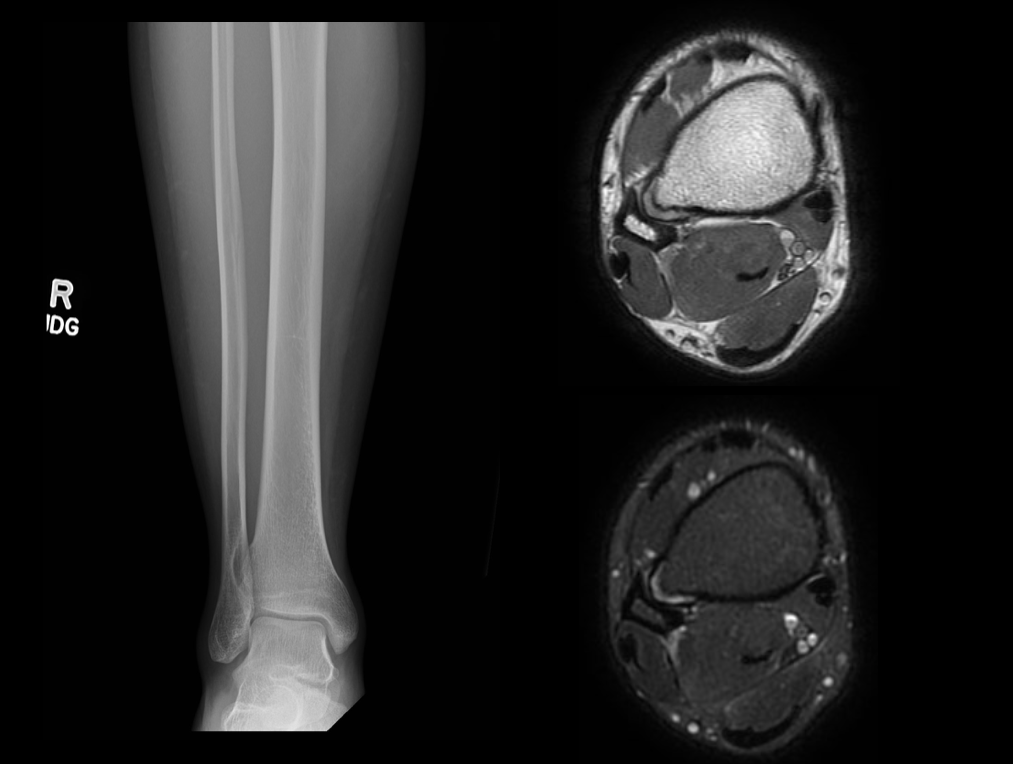
Osteochondromas are surface lesions. They are usually located at the metaphysis with characteristic cortical and medullary continuity of the parent bone and a cartilaginous cap.[41] The lesions may be sessile (broad base) or pedunculated (narrow stalk). Pedunculated lesions grow away from the adjacent joint. The characteristic cartilage cap is not easily assessable on radiographs as it is radiolucent and involutes at skeletal maturity. Sessile lesions bear a higher risk of malignant transformation.[18]
Radiographic findings concerning malignant transformation include an increase in size, changes in morphology, periostitis, or new indistinctness of the cortical margins. Lesions of the flat bones (scapula, pelvis, spine) are often indeterminant on radiographs and require further imaging to demonstrate the corticomedullary continuity.[1][2][3][12][23] See Images. Humerus Radiograph, Osteochondroma and Osteochondroma on AP Radiograph.
CT Scan or MRI
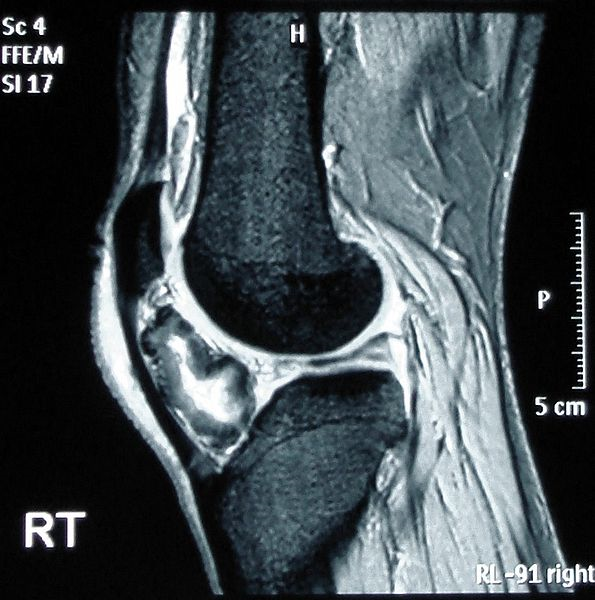
A CT scan or MRI can further help delineate the lesion. This form of imaging is indicated in patients with radiographic features suggestive of malignant transformation.[42] The cartilage cap will demonstrate intermediate to high signal on T2- and proton density (PD)–weighted images. Cartilage caps are generally thicker in skeletally immature patients (range 1 to 3 cm). In skeletally mature patients, the cartilage cap is generally only a few millimeters. Cartilage caps greater than 2 cm, especially in skeletally mature patients, are concerning for malignant transformation and require tissue sampling.[1][33]
MRI
MRI can help further assess associated complications such as overlying bursae, which present well on T2- and PD fat-suppressed as well-defined hyperintense fluid collections with or without bursitis. MRI is also useful to assess displacement or impingement on neurovascular structures. In patients with neurologic findings, the involved nerve may be displaced, enlarged, or demonstrate a hyperintense T2/PD signal. Additionally, the musculature innervated by the affected nerve may demonstrate edema (acute denervation injury), fatty infiltration (chronic denervation injury), or both.[1] See Image. Osteochondroma on MRI.
Bone Scintigraphy
This scan is generally not helpful, as both benign and malignant lesions may demonstrate increased radiotracer activity.[1]
Treatment / Management
Nonoperative Management
Nonoperative management is usually indicated for small solitary asymptomatic lesions. Most patients with HME do not require surgical intervention until skeletal maturity. No medical therapy for osteochondromas exists, although biological therapy might be available in the future.[19] One of the emerging potential treatments for HME is RARy agonists. RARy is a nuclear receptor and acts as a transcription factor as well. RARγ has a pivotal role in cartilage development and growth. The administration of RARy agonists has been reported to inhibit heterotopic ossification in mice and rats, and several other studies demonstrated its role in reducing the number of osteochondromas that developed in mice.[15][16][17][43]
Operative Management
Operative management might involve marginal excision of the lesion from its base (allowing removal of the stalk and the cartilage cap). However, in the pediatric population, delaying the surgery until skeletal maturity is recommended as the recurrence rate is higher in the immature skeleton.[44][45] Additionally, extra care should be considered when dealing with tumors close to the growth plate
Surgical Excision
Surgical excision is usually indicated for symptomatic lesions, aesthetically displeasing lesions, or lesions with suspicious imaging features such as irregular or indistinct margins, focal areas of radiolucency, bony erosions, or destruction, and lesions with uncertain diagnosis.[4][18][19][20][21] In spinal osteochondromas, surgical excision is an indication in radiculopathy, myelopathy, or vascular compression cases.[46][47] Additionally, surgical excision is indicated in cases of HME where the lesions are near the joints, resulting in restriction of the joint's range of motion or joint dislocations or subluxations.[1][23][33] Surgical excision is obligatory indicated in HME than solitary osteochondroma due to the higher risk of malignant transformation and severe bony deformities.[2][18][20][33]
Wide Surgical Excision
This treatment is indicated for secondary chondrosarcoma, a similar treatment that would be offered for a typical chondrosarcoma.
Reconstructive Options
Reconstructive options such as bone grafting and internal fixation could be indicated in cases where complete resection was undertaken (eg, in the pelvis).[19]
Differential Diagnosis
The differential diagnosis for osteochondroma includes both benign and malignant lesions.
Subungual Exostosis
Also referred to as Dupuytren exostosis, this is a common lesion of unknown etiology, thought to arise secondary to prior trauma or infection. The lesions classically arise from the dorsal aspect of the distal phalanx near the nail bed. The lesions may be painful with associated skin ulceration. Like osteochondromas, subungual exostosis is a surface lesion. However, there is no medullary continuity. Location is also a key distinguishing feature.
Dysplasia Epiphysealis Hemimelica
Also known as Trevor disease, this is a rare process with the development of multiple osteochondromas from the epiphysis, most commonly of the lower extremities. There is a 3:1 male-to-female predominance. The process is similar to HME, presenting in young patients secondary to altered gait, osseous deformity, or palpable mass. There are no reports of malignant degeneration in the literature.
Turret Exostosis
Turret exostosis is an extracortical mass on the back of the middle or proximal phalanx with no medullary continuity.
BPOP
Also known as Nora lesion, this surface lesion most commonly involves the osseous structures of the hands and feet. Although the etiology is unknown, it is considered secondary to prior trauma. The lesion does not have medullary continuity, and there is no reported risk of malignant degeneration.
Parosteal Osteosarcoma
This is a subtype of osteosarcoma arising from the surface of long bones. Radiographs demonstrate a large, lobulated, dense osseous mass without medullary continuity; however, the lesion can infiltrate the medullary space in advanced stages. Parosteal osteosarcoma most commonly arises from the metaphysis of the long bones, with the posterior margin of the distal femur the single most common location.
Juxtacortical Chondroma
This is a surface lesion that most commonly results in the saucerization of the adjacent cortex with associated periosteal reaction. Juxtacortical chondroma is more common in patients aged 20 to 40.
Subperiosteal Dematoma
This is a surface lesion with a smooth superficial cortical margin with an elliptical shape arising in patients with a history of prior trauma. There is no medullary continuity; centrally, the lesion may demonstrate heterogeneity with cystic areas, mineralization, or fat.[1][13][14][22][14][23]
Prognosis
The solitary form of osteochondroma has a good prognosis, with malignant transformation occurring in less than 1% of the patients. The majority of solitary lesions are small and asymptomatic. Simple excision of juxta-articular osteochondroma would improve the range of motion of the adjacent joint.
In cases of HME, approximately 5% to 10% of them can develop secondary chondrosarcoma, although spontaneous regression of the exostoses has been reported in 30% of the cases of HME.[19] Reports of the prevalence of malignant transformation are as high as 25% in HME; however, more recent studies suggest 3% to 5%. As previously described, patients with HME can have severe osseous deformities, which may affect their activities of daily living (ADL).[1] An extremely low risk of recurrence has been reported for cases that were completely resected with no remnants of the cartilage cap and perichondrium.[20][46][48]
Complications
Complications of osteochondroma can range from simple cosmetic concerns to serious neurovascular complications and malignant transformation. Complications fall into three categories: cosmetic deformity, mechanical effect, and malignant transformation. Major and minor complications have been reported in 4.7% and 7% of patients, respectively.[49]
Cosmetic Deformity
Painless swelling is the most common complaint that triggers patients to seek medical advice. The osseous deformity is often more severe in HME than in solitary osteochondroma.[1][13][23]
Mechanical Effect
Mechanical effects can result in impingement and repetitive mechanical compression on adjacent neurovascular structures. Since the knee is the most common location of an osteochondroma, there can be vascular complications.
Vascular Complications
These can include a pseudoaneurysm of the popliteal artery or even a true aneurysm, vascular compression, and arterial or venous thrombosis, all of which can compromise the blood supply to adjacent structures.
Neurological Complications
Depending on the lesion site, Neurological complications can result in peripheral neuropathy (eg, common peroneal nerve palsy with atrophy of the anterior and lateral leg compartment musculature) and radiculopathy or more serious spinal stenosis.[1][13][23][50][51][52]
Malignant Transformation
Malignant transformation is estimated to be 1% in solitary lesions and up to 3% to 5% in HME. As previously described, an increase in size or change of radiographic appearance should raise the suspicion of malignancy. Suspicious signs of malignancy on the radiograph include surface irregularity, areas of lucency and heterogeneous mineralization, and a thick cartilage cap (greater than 2 cm). Chondrosarcoma is the most common form of malignant transformation; however,
Complications of Surgical Excision
The rate of surgical-related complications for elective excision is higher than the rate of the tumor. This has been reported to be 12.5%, with neuropraxia being the most common, followed by artery laceration, compartment syndrome, and fibula fracture.[1][53][54][55]
Deterrence and Patient Education
Osteochondroma is a benign bony tumor that has a low risk of malignant transformation with an estimated risk of 1% for solitary lesions and up to 3% to 5% for HME.[1] Osteochondroma is usually asymptomatic and managed with observation. The evaluation mainly relies on conventional radiographs, with MRI reserved for symptomatic patients or lesions involving flat bones.
Symptomatic osteochondromas management is via surgical excision. Imaging features concerning for malignant transformation should prompt surgical removal or tissue sampling. Since the hereditary form of the bone tumor (HME) carries a higher risk of malignant transformation, regular follow-up is necessary.[1]
Enhancing Healthcare Team Outcomes
Osteochondroma is a benign bony tumor that is mainly asymptomatic and thus usually discovered incidentally. Although managing osteochondroma is the duty of orthopedic surgeons, interprofessional collaboration is a must for optimal patient care. Initial detection of osteochondroma may be found by the ER physician, radiologist, nurse practitioner, or family physician incidentally while investigating for another complaint or a complaint related to osteochondroma itself. A definitive diagnosis cannot be achieved without the participation of the pathologist and radiologist to confirm osteochondroma and exclude malignant features.
Symptomatic osteochondromas or those with suspicious malignant features will require surgery to excise the tumor. Performing any surgical procedure is a great example that demonstrates teamwork and cooperation among different healthcare professions, beginning with the preoperative phase that prepares the patient for the surgery. This phase is mainly the anesthesiologists' role in assessing the patient's fitness for surgery. The medical team may also be involved in the preoperative phase if the patient has any known medical illness or due to a newly discovered issue during the preoperative investigation.
During the operation, the orthopedic surgeon, anesthesiologist, nurses, and many others will collaborate to perform the surgery. Pharmacists can also weigh in with medication needed for the procedure, as well as postsurgical pain control and possible antibiotics, making recommendations to the team, monitoring drug interactions, and cautioning other team members regarding potential adverse events. Complications associated with osteochondroma may require additional specialties, including a neurosurgeon and vascular surgeon, to manage neurovascular complications. Finally, postoperative care and monitoring is an essential phase in which nurses and surgeons who performed the surgery work diligently to reduce postoperative complications.
Diagnosing and managing osteochondroma necessitates the collaboration of different healthcare professions, and achieving optimal care by the orthopedic surgeon alone is impossible. Osteochondroma diagnosis and management is an interprofessional team approach, including physicians, specialists, specialty-trained nurses, pharmacists, and possibly physical therapists, collaborating across disciplines to achieve optimal patient results.