[1]
Najera E, Truong HQ, Belo JTA, Borghei-Razavi H, Gardner PA, Fernandez-Miranda J. Proximal Branches of the Anterior Cerebral Artery: Anatomic Study and Applications to Endoscopic Endonasal Surgery. Operative neurosurgery (Hagerstown, Md.). 2019 Jun 1:16(6):734-742. doi: 10.1093/ons/opy308. Epub
[PubMed PMID: 30649510]
[2]
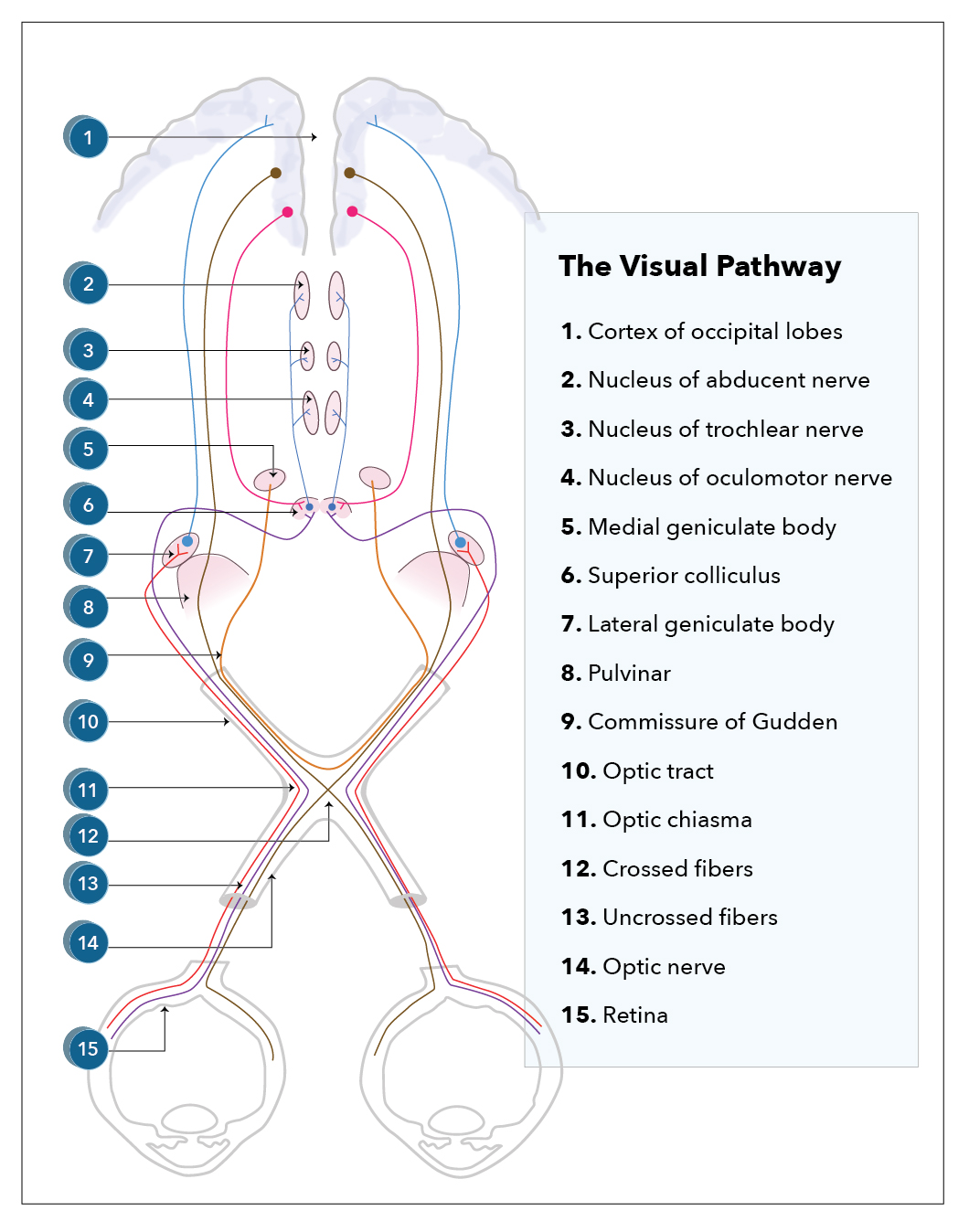
Costello F. The afferent visual pathway: designing a structural-functional paradigm of multiple sclerosis. ISRN neurology. 2013:2013():134858. doi: 10.1155/2013/134858. Epub 2013 Nov 6
[PubMed PMID: 24288622]
[3]
Murcia-Belmonte V, Erskine L. Wiring the Binocular Visual Pathways. International journal of molecular sciences. 2019 Jul 4:20(13):. doi: 10.3390/ijms20133282. Epub 2019 Jul 4
[PubMed PMID: 31277365]
[4]
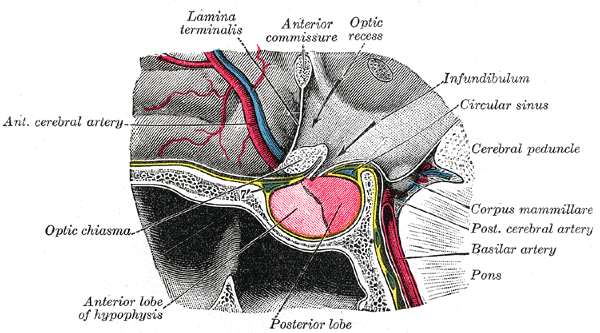
Gulsen S, Dinc AH, Unal M, Cantürk N, Altinors N. Characterization of the anatomic location of the pituitary stalk and its relationship to the dorsum sellae, tuberculum sellae and chiasmatic cistern. Journal of Korean Neurosurgical Society. 2010 Mar:47(3):169-73. doi: 10.3340/jkns.2010.47.3.169. Epub 2010 Mar 31
[PubMed PMID: 20379467]
[5]
Astorga-Carballo A, Serna-Ojeda JC, Camargo-Suarez MF. Chiasmal syndrome: Clinical characteristics in patients attending an ophthalmological center. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2017 Oct-Dec:31(4):229-233. doi: 10.1016/j.sjopt.2017.08.004. Epub 2017 Sep 6
[PubMed PMID: 29234224]
[6]
Takizawa G, Miki A, Maeda F, Goto K, Araki S, Yamashita T, Ieki Y, Kiryu J, Yaoeda K. Relative Afferent Pupillary Defects in Homonymous Visual Field Defects Caused by Stroke of the Occipital Lobe Using Pupillometer. Neuro-ophthalmology (Aeolus Press). 2018 Jun:42(3):139-145. doi: 10.1080/01658107.2017.1367012. Epub 2017 Aug 31
[PubMed PMID: 29796045]
[7]
Costea CF, Turliuc Ş, Buzdugă C, Cucu AI, Dumitrescu GF, Sava A, Turliuc MD. The history of optic chiasm from antiquity to the twentieth century. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2017 Nov:33(11):1889-1898. doi: 10.1007/s00381-017-3564-1. Epub 2017 Aug 14
[PubMed PMID: 28808784]
[8]
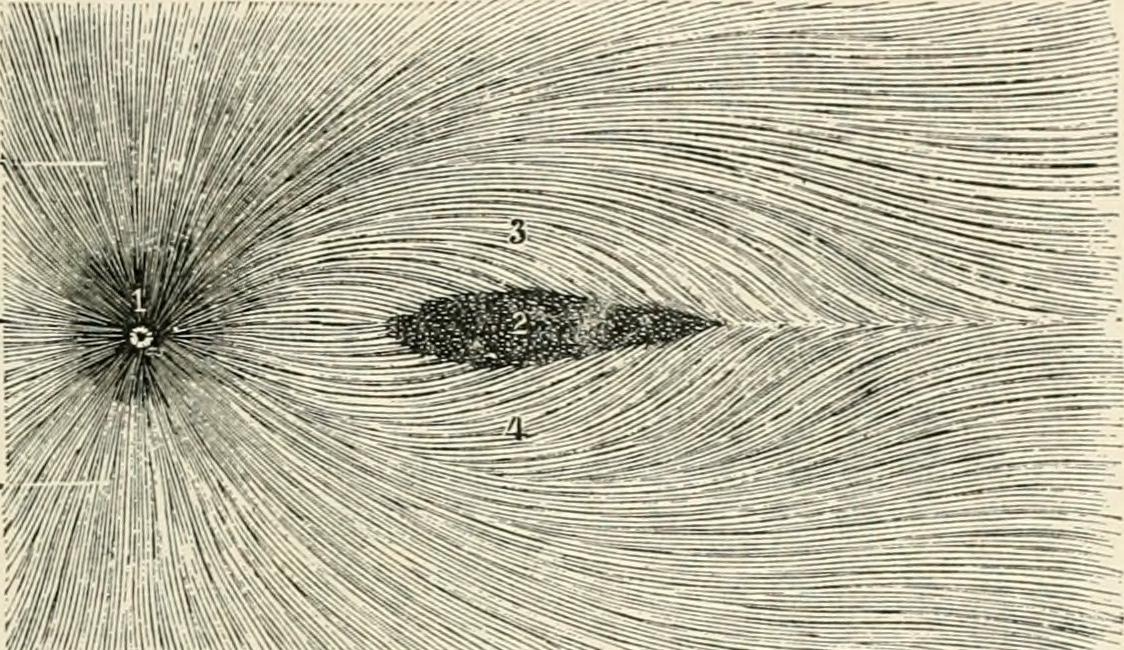
Jain NS, Jain SV, Wang X, Neely AJ, Tahtali M, Jain S, Lueck CJ. Visualization of Nerve Fiber Orientations in the Human Optic Chiasm Using Photomicrographic Image Analysis. Investigative ophthalmology & visual science. 2015 Oct:56(11):6734-9. doi: 10.1167/iovs.15-17443. Epub
[PubMed PMID: 26567784]
[9]
HOYT WF, LUIS O. Visual fiber anatomy in the infrageniculate pathway of the primate. Archives of ophthalmology (Chicago, Ill. : 1960). 1962 Jul:68():94-106
[PubMed PMID: 14449443]
[10]
Horton JC. Wilbrand's Knee: To Be or Not to Be a Knee? Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2020 Sep:40 Suppl 1(Suppl 1):S7-S14. doi: 10.1097/WNO.0000000000000988. Epub
[PubMed PMID: 32796340]
[11]
Grzybowski A, Kanclerz P. The Wilbrand's knee does not exist in the optic chiasm. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2018 Nov:34(11):2133. doi: 10.1007/s00381-018-3969-5. Epub 2018 Sep 8
[PubMed PMID: 30196392]
[12]
Prieur DS, Rebsam A. Retinal axon guidance at the midline: Chiasmatic misrouting and consequences. Developmental neurobiology. 2017 Jul:77(7):844-860. doi: 10.1002/dneu.22473. Epub 2017 Feb 12
[PubMed PMID: 27907266]
[13]
Larsson M. The optic chiasm: a turning point in the evolution of eye/hand coordination. Frontiers in zoology. 2013 Jul 18:10(1):41. doi: 10.1186/1742-9994-10-41. Epub 2013 Jul 18
[PubMed PMID: 23866932]
[14]
Erskine L, Herrera E. Connecting the retina to the brain. ASN neuro. 2014:6(6):. doi: 10.1177/1759091414562107. Epub 2014 Dec 12
[PubMed PMID: 25504540]
[15]
Mncube SS,Goodier MD, Normal measurements of the optic nerve, optic nerve sheath and optic chiasm in the adult population. SA journal of radiology. 2019;
[PubMed PMID: 31754545]
[16]
Wagner AL, Murtagh FR, Hazlett KS, Arrington JA. Measurement of the normal optic chiasm on coronal MR images. AJNR. American journal of neuroradiology. 1997 Apr:18(4):723-6
[PubMed PMID: 9127037]
[17]
Polat SÖ, Öksüzler FY, Öksüzler M, Uygur AG, Yücel AH. The determination of the pituitary gland, optic chiasm, and intercavernous distance measurements in healthy subjects according to age and gender. Folia morphologica. 2020:79(1):28-35. doi: 10.5603/FM.a2019.0058. Epub 2019 May 20
[PubMed PMID: 31106844]
[18]
Juenger V, Cooper G, Chien C, Chikermane M, Oertel FC, Zimmermann H, Ruprecht K, Jarius S, Siebert N, Kuchling J, Papadopoulou A, Asseyer S, Bellmann-Strobl J, Paul F, Brandt AU, Scheel M. Optic chiasm measurements may be useful markers of anterior optic pathway degeneration in neuromyelitis optica spectrum disorders. European radiology. 2020 Sep:30(9):5048-5058. doi: 10.1007/s00330-020-06859-w. Epub 2020 Apr 26
[PubMed PMID: 32335748]
[19]
Parravano JG, Toledo A, Kucharczyk W. Dimensions of the optic nerves, chiasm, and tracts: MR quantitative comparison between patients with optic atrophy and normals. Journal of computer assisted tomography. 1993 Sep-Oct:17(5):688-90
[PubMed PMID: 8370820]
[20]
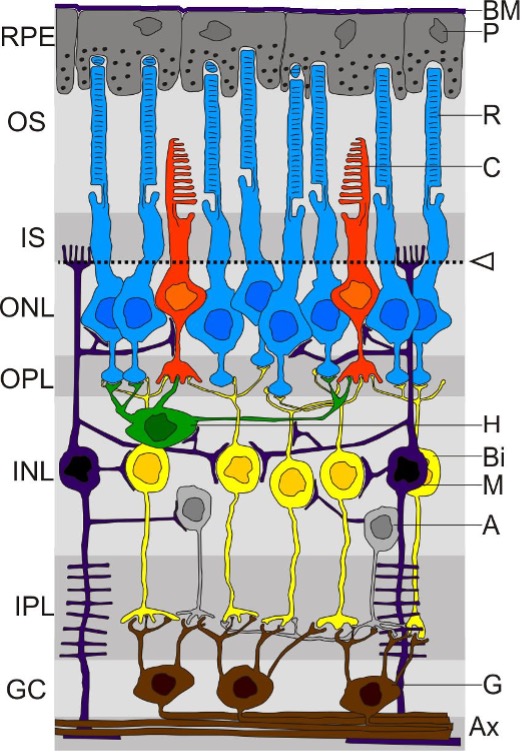
Edward DP, Kaufman LM. Anatomy, development, and physiology of the visual system. Pediatric clinics of North America. 2003 Feb:50(1):1-23
[PubMed PMID: 12713101]
[21]
Fuhrmann S, Zou C, Levine EM. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Experimental eye research. 2014 Jun:123():141-50. doi: 10.1016/j.exer.2013.09.003. Epub 2013 Sep 21
[PubMed PMID: 24060344]
[22]
Baykal D, Yilmazlar S, Fedakar R. A neurosurgical assessment of the blood supply in the optochiasmatic system: a cadaveric-anatomic study. Anatomical science international. 2021 Mar:96(2):294-300. doi: 10.1007/s12565-020-00595-3. Epub 2021 Jan 5
[PubMed PMID: 33400249]
[23]
Müller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera JP, Puget S. Craniopharyngioma. Nature reviews. Disease primers. 2019 Nov 7:5(1):75. doi: 10.1038/s41572-019-0125-9. Epub 2019 Nov 7
[PubMed PMID: 31699993]
[24]
Takahashi M, Goseki T, Ishikawa H, Hiroyasu G, Hirasawa K, Shoji N. Compressive Lesions of the Optic Chiasm: Subjective Symptoms and Visual Field Diagnostic Criteria. Neuro-ophthalmology (Aeolus Press). 2018 Dec:42(6):343-348. doi: 10.1080/01658107.2018.1438477. Epub 2018 Sep 11
[PubMed PMID: 30524487]
[25]
Raverot G, Burman P, McCormack A, Heaney A, Petersenn S, Popovic V, Trouillas J, Dekkers OM, European Society of Endocrinology. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. European journal of endocrinology. 2018 Jan:178(1):G1-G24. doi: 10.1530/EJE-17-0796. Epub 2017 Oct 18
[PubMed PMID: 29046323]
Level 1 (high-level) evidence
[26]
Shirane R, Hayashi T, Tominaga T. Fronto-basal interhemispheric approach for craniopharyngiomas extending outside the suprasellar cistern. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2005 Aug:21(8-9):669-78
[PubMed PMID: 16034620]
[27]
Ganna A, Dehdashti AR, Karabatsou K, Gentili F. Fronto-basal interhemispheric approach for tuberculum sellae meningiomas; long-term visual outcome. British journal of neurosurgery. 2009 Aug:23(4):422-30. doi: 10.1080/02688690902968836. Epub
[PubMed PMID: 19637015]
[28]
Kaptain GJ, Vincent DA, Sheehan JP, Laws ER Jr. Transsphenoidal approaches for the extracapsular resection of midline suprasellar and anterior cranial base lesions. Neurosurgery. 2008 Jun:62(6 Suppl 3):1264-71. doi: 10.1227/01.neu.0000333791.29091.83. Epub
[PubMed PMID: 18695546]
[29]
Maira G, Anile C, Albanese A, Cabezas D, Pardi F, Vignati A. The role of transsphenoidal surgery in the treatment of craniopharyngiomas. Journal of neurosurgery. 2004 Mar:100(3):445-51
[PubMed PMID: 15035280]
[30]
Sankhla SK, Jayashankar N, Khan GM. Extended endoscopic endonasal transsphenoidal approach for retrochiasmatic craniopharyngioma: Surgical technique and results. Journal of pediatric neurosciences. 2015 Oct-Dec:10(4):308-16. doi: 10.4103/1817-1745.174457. Epub
[PubMed PMID: 26962333]
[31]
Liu JK, Christiano LD, Gupta G, Carmel PW. Surgical nuances for removal of retrochiasmatic craniopharyngiomas via the transbasal subfrontal translamina terminalis approach. Neurosurgical focus. 2010 Apr:28(4):E6. doi: 10.3171/2010.1.FOCUS09309. Epub
[PubMed PMID: 20367363]
[32]
Griessenauer CJ, Raborn J, Mortazavi MM, Tubbs RS, Cohen-Gadol AA. Relationship between the pituitary stalk angle in prefixed, normal, and postfixed optic chiasmata: an anatomic study with microsurgical application. Acta neurochirurgica. 2014 Jan:156(1):147-51. doi: 10.1007/s00701-013-1944-1. Epub 2013 Nov 28
[PubMed PMID: 24287682]
[33]
Tantiwongkosi B, Mafee MF. Imaging of Optic Neuropathy and Chiasmal Syndromes. Neuroimaging clinics of North America. 2015 Aug:25(3):395-410. doi: 10.1016/j.nic.2015.05.004. Epub
[PubMed PMID: 26208416]
[34]
Kedar S, Ghate D, Corbett JJ. Visual fields in neuro-ophthalmology. Indian journal of ophthalmology. 2011 Mar-Apr:59(2):103-9. doi: 10.4103/0301-4738.77013. Epub
[PubMed PMID: 21350279]
[35]
Shin WJ, Song BJ, Kim JM. Junctional scotoma in giant cerebral aneurysm. Korean journal of ophthalmology : KJO. 2002 Dec:16(2):124-9
[PubMed PMID: 12546452]
[36]
Ho RW, Huang HM, Ho JT. The influence of pituitary adenoma size on vision and visual outcomes after trans-sphenoidal adenectomy: a report of 78 cases. Journal of Korean Neurosurgical Society. 2015 Jan:57(1):23-31. doi: 10.3340/jkns.2015.57.1.23. Epub 2015 Jan 31
[PubMed PMID: 25674340]
Level 3 (low-level) evidence
[37]
Fried I, Tabori U, Tihan T, Reginald A, Bouffet E. Optic pathway gliomas: a review. CNS oncology. 2013 Mar:2(2):143-59. doi: 10.2217/cns.12.47. Epub
[PubMed PMID: 25057976]
[38]
De Silva SR, Arno G, Robson AG, Fakin A, Pontikos N, Mohamed MD, Bird AC, Moore AT, Michaelides M, Webster AR, Mahroo OA. The X-linked retinopathies: Physiological insights, pathogenic mechanisms, phenotypic features and novel therapies. Progress in retinal and eye research. 2021 May:82():100898. doi: 10.1016/j.preteyeres.2020.100898. Epub 2020 Aug 26
[PubMed PMID: 32860923]
[39]
Grønskov K, Ek J, Brondum-Nielsen K. Oculocutaneous albinism. Orphanet journal of rare diseases. 2007 Nov 2:2():43
[PubMed PMID: 17980020]
[40]
Sami DA, Saunders D, Thompson DA, Russell-Eggitt IM, Nischal KK, Jeffrey G, Dattani M, Clement RA, Liasis A, Taylor DS. The achiasmia spectrum: congenitally reduced chiasmal decussation. The British journal of ophthalmology. 2005 Oct:89(10):1311-7
[PubMed PMID: 16170123]