Introduction
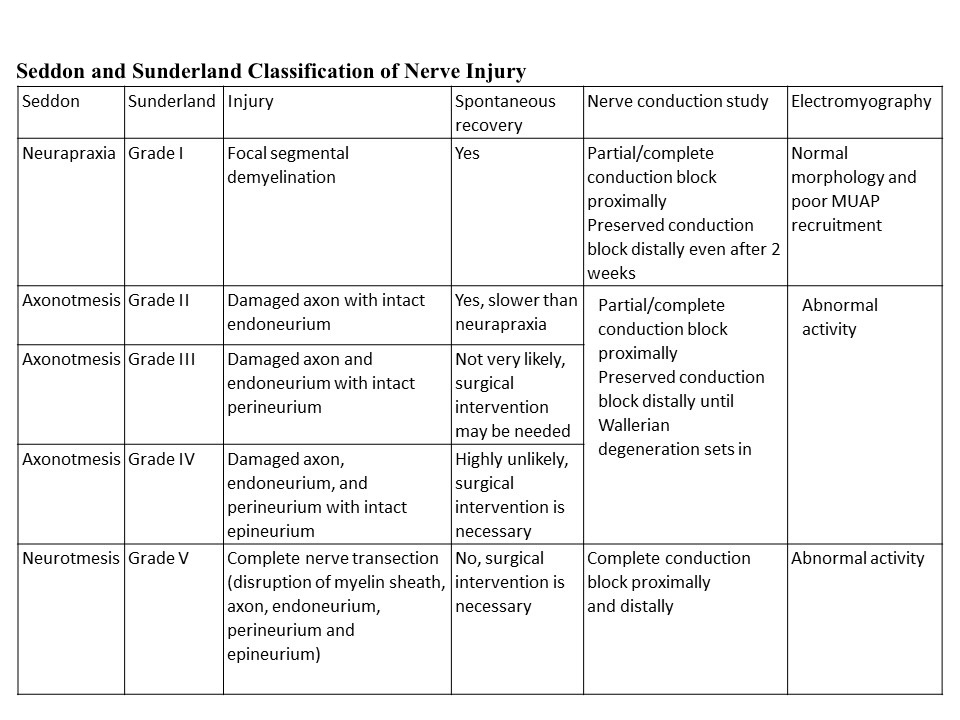
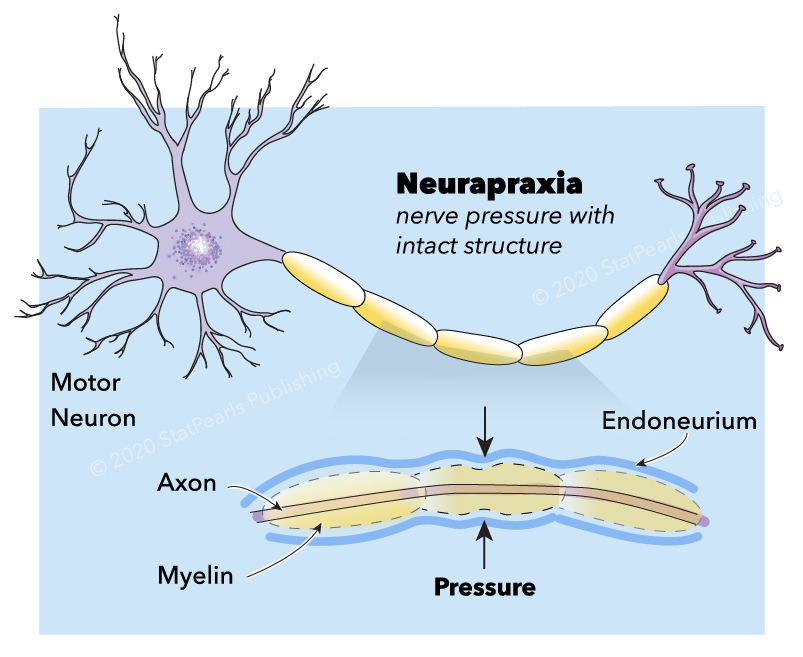
Neuropraxia is the mildest form of traumatic peripheral nerve injury. It is characterized by focal segmental demyelination at the site of injury without disruption of axon continuity and its surrounding connective tissues. This condition results in blockage of nerve conduction and transient weakness or paresthesia. Complete recovery is the expectation upon spontaneous remyelination—peripheral nerve injury (PNI) organizes into five categories that clinicians use today (see Image. Neurapraxia). Sunderland stratified and expanded Seddon's (1943) classification into Grade I, refers focal segmental demyelination; Grade II refers to damaged axon with intact endoneurium; Grade III refers to damaged axon and endoneurium with intact perineurium; Grade IV refers to the damaged axon, endoneurium, and perineurium with intact epineurium; and Grade V refers to complete transection (see Table. Seddon and Sunderland Classification of Nerve Injury).[1][2][3][4][5][6][7][8] Some authors also describe a sixth-degree peripheral nerve injury, which refers to the mixed pathology of injury.[5][3]
Neuropraxia, and PNI in general, can be secondary to trauma from sports, accidents, or improper positioning.[6][7][5] Injury to nerves results in motor or sensory loss, pain, or a combination of these, leading to significant morbidity or functional impairment.[9][3][7]
Structure and Function
Neuronal cells, glial cells, and stromal cells comprise the peripheral nervous system. The nerves are consist of different combinations of sensory, motor, and autonomic neurons. Motor and autonomic neurons are efferent neurons that receive signals from the central nervous system, while sensory neurons are afferent neurons that receive signals from specialized cell types (e.g., Pacinian corpuscles).[2] The cell bodies, or neurons, are located in the spinal cord. Their long cytoplasmic extensions are called axons.[10]
The stromal connective tissues are the endoneurium, perineurium, epineurium. They are important scaffold (mechanical and protective role) for the nerve and are essential in comprehending and classifying peripheral nerve injuries. Each myelinated or unmyelinated axon is enclosed by the endoneurium, which consists of collagen fibers. Axons are grouped together in bundles called a fascicle. The perineurium surrounds each fascicle and separates extracellular space from intrafascicular endoneurial space. It is composed of specialized myofibroblasts and intercellular tight junctions. The outermost stromal layer is the epineurium, which is rich in collagen fibers. It is composed of two parts, the epifascicular and epineural epineurium. The epifascicular epineurium or internal epineurium encases and separates fascicles while the epineural epineurium or external epineurium is more superficial and surrounds the nerve trunk proper. The external epineurium defines a nerve anatomically.[2][7][10][11][12][13] The extensibility of the epineurium is responsible for nerve gliding necessary to accommodate joint motion. A defect on this property of the epineurium results in nerve traction injury.[14] External to the epineurium is the mesoneurium or perineurium. It is a loose connective tissue that stabilizes the nerve's position and contains the blood supply to the nerve.[7][10]
Schwann cells are glial cells that enclose nerves in a layer of myelin. It also releases neurotrophic factors such as nerve growth factor (NGF), which provide trophic support. The myelin enhances conduction velocity by restricting the sites of ionic transfer in the axon to the nodes of Ranvier; this results in saltatory conduction, an expedient, "jumping" action potential propagation. There are myelinated and myelinated nerve fibers. The most heavily myelinated fibers are type Aα (efferent to muscles, for the motor function), followed by Type Aβ (afferent from skin and joints, for tactile & proprioception), type Aγ (efferent to muscle spindles, for muscle tone), type Aδ (from afferent sensory nerves; for pain, cold, temperature and touch) and the least myelinated is type B (preganglionic sympathetic, for various autonomic functions). Type C is unmyelinated and is responsible for multiple autonomic functions, pain, temperature, and touch.[2][10][7]
Embryology
The peripheral nervous system (PNS), like the central nervous system, arise from the neural plate. The cells at the neural plate margin give rise to neural crest cells. Neural crest cells migrate extensively throughout the embryo, which contributes to the majority of glial cells and neurons in the PNS. At the cranial level, the PNS has a dual origin, the ectodermal placode, and cranial neural crest cells. The placodes generate the "sense" organs (ears, nose, the lens of the eye) and cranial sensory ganglia. Caudally, the entire peripheral nervous system derives from the neural crest cells. The neural crest cells contribute to sympathetic ganglia and the dorsal root of the PNS. The dorsal root ganglia form bilaterally beside the developing spinal cord. It is sensory and innervates the skin and organs for proprioception, injury, and temperature. Other neural crest cells migrate ventrally, forming the sympathetic chain ganglia, which innervate various organs along the trunk.[15]
Blood Supply and Lymphatics
Within the nerve fascicles are the endoneurial microvessels and investing perineurium. Together, they form the blood-nerve barrier (BNB).[11][16][14] The microvessels are non-fenestrated endothelium derived from the anastomosing vasa nervorum complex in the epineurium penetrating the perineurium. It resides in the endoneurium and provides blood to axons.[2][11] Meanwhile, the perineural cells also have tight junctions that contribute to forming the blood-nerve barrier.[7][11] These two restrict the permeability of the fluid and protect the endoneurial microenvironment from sudden changes in concentration. The hypothesis is that the endoneurial homeostatic mechanism modulates the milieu inside the Schwann cells and peripheral axons by facilitating normal axonal signal transduction (sodium/potassium flux) in the peripheral nervous system.[11][12] Unlike most extracellular spaces, this uniquely regulated microenvironment lacks a lymphatic circulation.[12]
Meanwhile, the epineurial macro vessels are composed of anastomosing plexus derived from the extrinsic blood vessel supplying the nerve.[12][11] The anastomosis between the perineurial and epineurial circulation occurs at different levels in the perineurium. The longitudinally-oriented blood vessels penetrate the cellular layers obliquely to connect with endoneurial cells.[12]
Surgical Considerations
Neurapraxia, as a complication of perioperative injuries, is usually via compression. Inappropriate perioperative position and/or poor padding are the main culprits. Examples include ulnar nerve damage in the park bench position, due to increased pressure and excessive flexion at the level of the elbow; the radial nerve suffers injury due to compression between the humerus and the edge of the operating table [17]; the sciatic nerve and peroneal nerves are injured in the lithotomy position due to the hyperflexion of the hip causing sciatic nerve traction and peroneal nerve compression against the head of the fibula, respectively.[17][18] Two studies denoted rare complications of airway manipulation (e.g., supraglottic airway instrumentation, endotracheal intubation, laryngeal mask insertion), lingual nerve neurapraxia, and greater palatine nerve neurapraxia.[19][20]
Local anesthesia (LA) can cause neurapraxia through different mechanisms. One way is by causing vasoconstriction, which leads to nerve ischemia. It can also produce chemical nerve injury.[17][7] This happens following LA administration, due to difference in osmolarity, the endoneurium fluid changes from hypertonic to hypotonic, leading to edema and increases intrafascicular pressure. Nonetheless, even high extrafascicular concentrations can damage nerves. Mechanically, LA administration can also cause neurapraxia through nerve compression by creating high pressures surrounding the nerve as well as neurotmesis by needle perforation.[7] Lastly, LA can give rise to an inflammatory complication, causing scar tissue formation and subsequent nerve entrapment.[14][7]
Clinical Significance
The true incidence of neurapraxia is challenging to assess due to the following factors: It is reported as peripheral nerve injury in general, and PNI are almost always missed, underreported, and underestimated.[6][7][5] The annual incidence of PNI is 13 to 23 per 100,000 persons per year.[3][21] In patients admitted to trauma centers, estimates are that 2 to 3% of patients have PNIs.[6] In sports-related injuries, PNIs occur in <0.5% of injuries. [4] The incidence of perioperative nerve injuries varies depending on the operation. It ranges from 1% in hip arthroplasty to 75% in anterior cruciate ligament reconstruction cases. An extremely common nerve injury (88%) is an injury to the lateral femoral cutaneous nerve when performing procedures with anterior access.[7]
Neurapraxia typically results from mild traction or compression of the nerve. On the other end of the spectrum, neurotmesis result from transection injuries such as laceration, penetrating injuries, or gunshot wounds.[2] There are diverse ways of how the peripheral nerve can be compressed or stretched. It could be due to casual incautious positioning (e.g., hanging one's arm over a chair) [2], sports injury, and accidents such as vehicular accidents or falls.[4][6][22] In the hospital, various circumstances can also cause nerve compression or traction. For example, improper padding and poor perioperative positioning, use of tourniquets, surgical retractors, and airway manipulation.[18][17][7][17][23][24][19][20] Inflammation can also engender scar tissue formation and subsequent nerve entrapment; this can be due to local anesthetics, disinfectants, perineural hematoma, and postsurgical inflammatory neuropathy.[7]
Based on a retrospective study, from 1989 to 2014, of 1124 patients (1418 nerve lesions), results showed the most common cause of PNI is vehicular accidents (46.4%), which affected mostly young men. Meanwhile, of the 866 cases with identified etiology, other causes are: penetrating trauma (23.9%), falls (10.9%), gunshot wounds (6.6%), car accidents involving pedestrians (2.7%), sports-related (2.4%) and miscellaneous (7.2%).[6]
Most peripheral nerve compression injuries fall under Grade I or neurapraxia, which commonly occur in areas where the nerve passes through narrow anatomical openings. Compressions can be acute or chronic. Proposed mechanisms that appear to give rise to nerve compression and traction injuries include narrow openings and superficial nerve locations, which makes the nerve and its blood supply vulnerable to mechanical compression,[2][4][25][17][7][22][23][26][19][20][27][8][24][28] Increased pressure in a confined compartment (i.e., compartment syndrome),[4][9][29] traction of surrounding tissue, synovial tissue hypertrophy, tenosynovitis, hematoma, aneurysm, pseudoaneurysm, and edema or high-volume local anesthetic injection proximal to the nerve or blood vessel.[25][14][7][9] Traction results from a defect in the extensibility of epineurium, which is responsible for nerve gliding, which is necessary to accommodate joint motion.[14]
Patients with peripheral nerve injuries usually present with sensory or motor function disorders.[4][7] It is essential to elicit the precipitating event (e.g., accidents, sports, recent surgeries) and timing of the symptoms. The onset of neurologic symptoms after the injury may be acute or delayed, hours to days and weeks, respectively. The acute-onset is associated with direct damage to the nerve, while the delayed-onset is associated with inflammation and edema.[7]
In these cases, a good knowledge of anatomy is beneficial.[4] The history should be thorough and the neurological examination, complete and meticulous, to obtain an accurate diagnosis and administer the appropriate management. Test for motor function and deranged sensation should include and follow specific nerve innervations. Depending on the nerve involved, neurologic findings include sensory impairment such as pain, allodynia, hyperesthesia, hypoesthesia, paresthesia, motor impairment such as weakness, atrophy in chronic cases, hyporeflexia. [4] Results of the neurologic examination serve to identify the type of injury, the location of the lesion, and the degree of injury of the sensory, motor, or both sensory-motor impairment, which is of prognostic value.[17][18][4][22]
Differential diagnosis includes peripheral neuropathies due to diabetes, hypothyroidism, alcohol, and malnutrition, radiculopathy, myelopathy, spinal cord trauma or infarction, muscle diseases.[18]
The history and neurologic examination can diagnose a specific nerve involvement (PNI); however, the severity of the nerve damage is difficult to distinguish. The electrodiagnostic study, electromyography (EMG), and nerve conduction studies(NCS)), can differentiate neurapraxia, axonotmesis, and neurotmesis. It can localize the lesion and help in prognostication (Table 1).[30][3][4][6][17][7][5] However, 3rd and 4th-degree injuries are distinguished only indirectly.[3]
Blood investigations (complete blood count, liver and renal function, blood glucose, erythrocyte sedimentation rate, thyroid-stimulating hormone, and vitamin B12) should be requested to evaluate non-traumatic causes of peripheral nerve injury. [18] The further investigation includes targeted serum and plasma analysis, magnetic resonance imaging (MRI), somatosensory evoked potentials (SEP), and nerve biopsy. Although rarely used, the ultrasound can provide data regarding the dynamic connection of the joint and nerve during movement and likewise localize the compression site.[18][4]
The management in peripheral nerve injury has four goals: to identify the lesion, to correct the pathology, to alleviate the symptoms, and to inform, reassure, and support the patient.[18][5][17] The ultimate goal is the restoration of function of the target organs to their original capacity, both in motor and sensory function.[17][31]
Initial treatment includes rest, physical rehabilitation, orthotic measures (e.g., splints, foot care, limb supports), avoidance of the aggravating activity, and pain relievers for neuropathic pain (e.g., analgesics, antidepressants, anticonvulsants, corticosteroids, anesthetics).[3][18][4][17][8][28] For athletes, it may be necessary to change the method of training and using sports equipment. Supportive braces and specialized gloves may also be helpful.[4] Reversible causes should be corrected. For example, in cases where the peripheral nerve injury is due to nerve compression due to hematoma, urgent surgical decompression may be considered [18][9]; in nerve compression secondary to fracture, surgical management may be required [27]; in compartment syndrome, fasciotomy.[4][9] If there is no clinical improvement in 6 weeks, an electrodiagnostic study is useful to evaluate for the degree of nerve injury.[3][4]
As the mildest form of peripheral nerve injury, neurapraxia has an excellent prognosis. Complete recovery due to axon remyelination is the expectation within days to weeks.[5][4][17][7][8]
Other Issues
Nerve conduction studies are performed by stimulating a nerve using an external electrode in two different locations with its receiving electrode placed over the muscle or skin supplied by the nerve. The motor response is called compound muscle action potential (CMAP). CMAP reflects the sum of motor unit potentials stimulated. The amplitude is directly proportional to the number of depolarized axons. When the amplitude of the CMAP no longer changes, this means that all the axons are stimulated. On the other hand, the sensory response is called sensory nerve action potential (SNAP). SNAPs aid in determining whether the lesion is post-ganglionic or pre-ganglionic, which ultimately helps in localizing proximal lesions. The parameters measured in NCS are amplitude, latency (the time it takes to generate a response [i.e., CMAP or SNAP] from the time of administering the stimulus), and conduction velocity. In neurapraxia, CMAP and SNAP can be elicited distal to the injury. Proximally, NCV shows partial or complete conduction block, varying degrees of decreased CMAP and SNAP amplitude, and reduced conduction velocity. When remyelination is complete, these changes improve completely or partially.[7][5][30]
Meanwhile, EMG is performed by directly inserting a needle electrode in the muscle that is at rest and during contraction, which can record action potentials in the muscle. It helps determine the chronicity of injury and differentiate if the cause of weakness is neuropathic or myopathic. It is used to detect abnormal spontaneous activity at rest, insertional activity, and motor unit action potential (MUAP) during voluntary contraction. The abnormal spontaneous activity aids in determining the onset of injury, the probable location, nature, and severity of the lesion. It includes fibrillation (FIB) and positive sharp waves (PSW). In neurapraxic lesions, the muscle does not reveal any abnormal spontaneous activity (i.e., absent FIB and PSW), and there are normal morphology and poor MUAP recruitment on voluntary contraction.[7][5][30]
The electrodiagnostic study can be falsely reassuring from the time of injury up to two weeks because, at this time, Wallerian degeneration (i.e., in axonotmesis) is not yet complete.[30][8] CMAP and SNAP do not decrease for 2 to 3 days. CMAP amplitudes reach the lowest point 9 days after the insult, while SNAP takes 10 to 11 days post-injury. Within this time, stimulation proximal to the injury will not differentiate demyelination and axon loss, and stimulation distal to the injury may not reveal the pathology. Hence, it is logical to perform electrodiagnostic studies 10 to 14 days after the precipitating event. When Wallerian degeneration sets in after 10 to 12 days, there is a failure to record CMAP and SNAP with distal stimulation, which remains preserved in neurapraxia. In time, the proximal conduction block with preserved distal CMAP and SNAP suggests neurapraxia. Electrodiagnostic studies can still be done immediately after the insult, but it serves only to determine any pre-existing pathology.[5][30][7] In several cases, the initial tests are conducted three weeks after insult to obtain a reliable result.[30][7][8] In this period, when the EMG and NCS cannot detect the peripheral nerve injury, MRI can show early damage.[4]
In the retrospective study of 1124 cases, neurapraxia electrodiagnostic studies showed absent or rare FIB and PSW, normal MUAP morphology with reduced recruitment, and normal or reduced/absent SNAP or CMAP.[6]
There have been recent advances made in the diagnostic evaluation of peripheral nerve injuries. The high-resolution ultrasound imagings help in comparing the cross-sectional area of the concerned nerve with that of its healthy counterparts.[32][33] Additionally, it can be performed as a bedside procedure and also provides real-time assessment of not only the nerves but also helps in ruling out other associated soft tissue injuries. It also helps in the assessment of vascular integrity.[34] However, it is operator dependent, has a learning curve, and also requires some duration of time for the axonal wasting and thereby the cross-sectional area to be significantly appreciatable in the ultrasound.
Similarly, studies have verified the highest sensitivity of the proton density fat-suppressed MR sequences in ruling out peripheral nerve injury in comparison to that of the high-resolution ultrasound studies.[34] There will be absent or very minimal high densities seen in the fat-suppressed T1 and the T2 MR sequences in cases of neurapraxia.[34]
Moreover, the usage of the fractional anisotropy, that measures the axonal density as well as the diffusion pattern along the nerve fibers, in conjunction with the tractography, helps in the earliest assessment of the continuity of the fiber tracts as well as visualization of any wasting of the fascicles, in comparison to that of their healthy counterparts.[35]