Introduction
Neurobiology is a vast and rapidly expanding field with significant clinical and technological implications. Far-reaching questions, such as what composes human personalities and the development of consciousness, are boiled down to quantitative processes. At the root of this field is the neuron and how information gets transferred throughout the body via electrochemical signals. This article will cover the mechanism of such communication as a fundamental review of nerve physiology.
Cellular Level
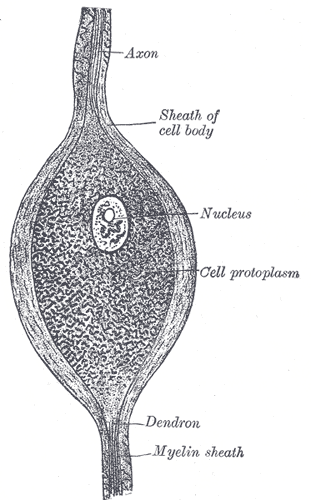
A neuron consists of a cell body called the soma, and at least one branch called a neurite. Neurites that relay signals away from the soma are called axons, and neurites that r relay signals toward the soma are called dendrites. The soma contains organelles similar to other cell bodies, such as a nucleus, mitochondria, and lysosomes. The rough endoplasmic reticulum of a neuron, which is termed Nissl substance, is exceptionally prominent because it must synthesize a large amount of membrane for the neurites.[1][2]
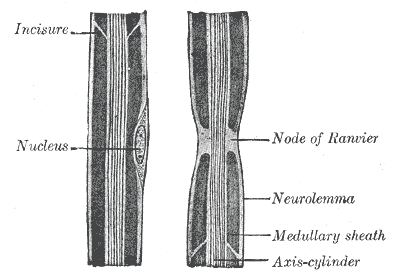
Most axons and long dendrites are coated with a protective covering called myelin, which is a membrane that acts as an electrical insulator to increase the speed of conduction of impulses. Myelin is made when a non-neuronal support cell called a glial cell wraps itself tightly around a neurite, squeezing the glial cell cytoplasm towards the periphery, leaving only its cell membrane in the inner layer. Myelin does not cover the neurite completely but leaves gaps called nodes of Ranvier, which will be discussed further in the Mechanism section. In the central nervous system (CNS), myelin derives from glial cells called oligodendrocytes, which synthesize myelin bundles for many axons. In the peripheral nervous system (PNS), the source of myelin is specialized cells called Schwann cells, with each Schwann cell creating just one myelin bundle of an internode of a single axon.[3]
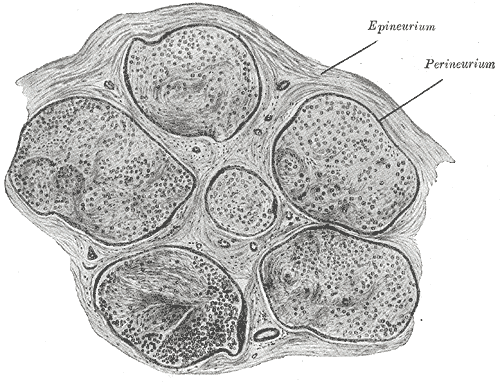
Axons of multiple neurons are bundled together into a structure called a nerve. Within a nerve, each individual axon is enclosed within a connective tissue layer called the endoneurium. The endoneurium creates endoneurial fluid, which protects the axon during times of injury. Many axons bundle together into a fascicle, and the covering fo each fascicle is a layer called perineurium. The outermost layer is the epineurium, which encloses multiple fascicles and blood vessels of the nerve.[1][4]
Development
Neurons form from ectodermal embryological derivatives. During embryogenesis, a region of the axial mesoderm forms the notochord, which lies ventral to the neuroectoderm. The notochord induces the folding of the neural plate into the neural tube, which becomes the neuroectoderm. CNS neurons derive from the neuroectoderm while PNS neurons(ganglion cells) and Schwann cells derive from neural crest cells, which border the neural plate. Neuron development ceases before birth, so any neuron that dies cannot be replaced, and all further changes to the nervous system are due to change in connectivity between existing neurons. Thus, after birth, the nervous system is characterized by plasticity, the ability to be shaped by experiences. Developmental processes such as neural migration, maturation, and synaptogenesis are under the influence of environmental factors such as sensory stimuli, relationships, hormones, and particular drugs.[5][6]
Function
The primary function of nerves is to send information throughout the body via electrochemical signals. Nerves classify into three functional categories: sensory neurons, motor neurons, and interneurons. Sensory neurons receive and interpret sensory stimuli, motor neurons relay messages to muscles or glands, and interneurons transmit signals between other neurons. Nerve fibers that send information from sensory neurons to the central nervous system are termed afferent nerve fibers, while nerve fibers that carry information away from the central nervous system, such as the motor neurons to the muscles, are termed efferent nerve fibers.[1][7]
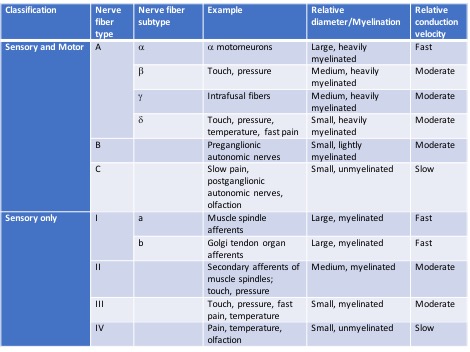
The sensory receptors are specialized regions or structures of the sensory neurons responsible for detecting all senses and performing many functions throughout the body. These receptors respond to changes in the environment by converting chemical or mechanical energy into action potentials to be transmitted towards the central nervous system. Different types of stimulus receptors mediate each of the five basic human senses (sight, hearing, smell, taste, and touch). There are many other types of receptors, including chemoreceptors, photoreceptors, baroreceptors, and proprioceptors, which detect chemicals, light, pressure, and movement, respectively. Nerve fibers have been categorized based on numerous parameters, including relative diameter and myelination, as outlined in the image below.[8][9]
Mechanism
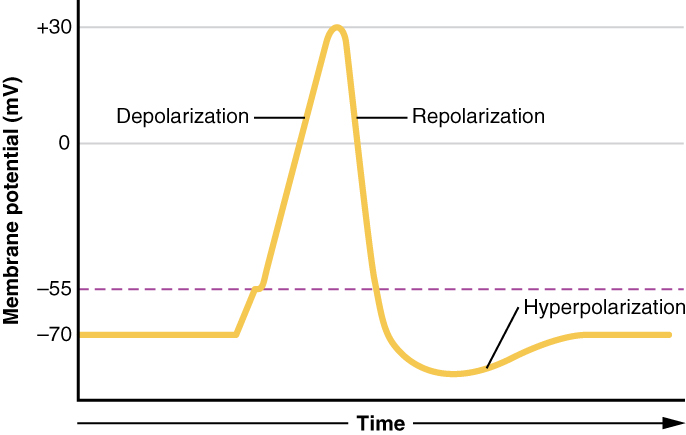
Nerve impulses get conveyed and transmitted via action potentials, which are brief changes in membrane potential. Neurons have a resting membrane potential around -60mV; this is due to a layer of negatively charged ions on the inner cell membrane of the neuron and a layer of positively charged ions on the outer cell membrane. This separation of charge results in electrical potential, which at rest (not during an action potential) is 60 mV more negative in the cell interior. This potential is generated from the movement of sodium, potassium, and chloride ions into and out of the cell down their concentration and electrical gradients, with the Na-K pump, actively pumping sodium and potassium against their concentration gradient to prevent concentration equilibrium. The potassium concentration inside a neuron is high, and sodium concentration is relatively low, so potassium ions are driven out of the cell by their concentration gradient while sodium ions are driven inside the cell - this is balanced by the electrical gradient as well, with the negatively charged cell interior pulling positively charged ions inside. The neuron cell membrane is most permeable to potassium due to a large - amount of potassium channels, so the resting cell membrane of the neuron is most affected by relative potassium concentrations.[10][11][12]
Upon stimulation, a change in sodium channel permeability or opening of the voltage-gated sodium channel causes sodium to rush into the cell following the concentration gradient of sodium. The membrane potential rapidly changes to approach zero and becomes positive, a process known as depolarization. This action potential only lasts a millisecond, but it spreads to adjacent sections of the axon, causing an electrochemical wave. Also, action potentials are an all-or-none phenomenon, meaning that the minimum threshold of depolarization must be met to trigger a full amplitude action potential, or none will occur at all.[10][11][12]
This depolarization process is mediated by the flow of ions across voltage-gated ion channels. A sufficiently strong depolarization event causes voltage-gated sodium ion channels to open, allowing sodium ions to flow across the cell membrane into the neuron. As this occurs, the membrane potential increases dramatically. Voltage-gated potassium channels also open, but only after a small delay. This allows potassium ions to flow outside of the neuron, away from the now more positively charged cell interior, which brings the cell membrane potential back down towards the original -60mV. The voltage-gated sodium channels, which were activated first, also close first, resulting in a brief period where sodium channels close while potassium channels remain open, resulting in a temporary hyperpolarization period where the membrane potential is below -60 mV until the voltage-gated potassium channels inactivate as well. These voltage-gated ion channels have a brief refractory period of inactivity after closing, where no amount of stimulus can reactivate them, which prevents redundant action potentials from occurring.[10][11][12]
Nerve impulses are dependent on the ability of the resulting action potential to propagate down the length of the axon without losing amplitude. This process differs in myelinated and unmyelinated axons. In unmyelinated axons, the original action potential results in the inflow of sodium ions. These sodium ions will be repelled from each other and attracted to the nearby more negative section of the axon, which causes depolarization there as well, thus opening of adjacent voltage-gated ion channels, allowing more sodium ions to enter, further depolarizing along the length of the axon. The speed of propagation is dependent on the rate of depolarization of the segment of axon in front of the action potential. This speed gets influenced by the concentration of the sodium channels and the diameter of the axon. The larger the axon, the less internal resistance there is to ion flow, thus there is greater conduction and faster nerve impulses. The presence of myelin greatly increases conduction velocity. Myelin decreases the capacitance, which is the ability to store charge, of the section of the axon it covers. If the section of the axon cannot store charge, more charge is spread along the rest of the axon, increasing the rate of depolarization and speed of propagation. Myelin is wrapped so tightly around the axon that ion flow across the axon membrane cannot occur, otherwise known as increased transmembrane resistance, which is why myelinated axons only develop action potentials at small gaps in myelin called nodes of Ranvier. Once an action potential develops at a node, the current travel quickly along the myelinated section to the next node, where another action potential gets generated. These jumps in action potentials in myelinated axons are termed saltatory conduction. Myelinated axons have developed specific characteristics to maximize effectiveness. Voltage-gated sodium channels are much denser at nodes of Ranvier and sparse to absent under the myelin sheath. This allows the neuron to focus its energy on opening and synthesizing channels in the nodes rather than wasting resources in the myelinated section where ions need to propagate along the axon.[10][11][12]
The properties of neurites allow conduction of action potentials along the length of the neuron. When a neural impulse reaches the end of the neuron, it must be transmitted to a neighboring neuron to communicate with the rest of the body. This process, called synaptic transmission, can occur electrically or chemically. Neurons that are close enough together can directly transfer ions from one neuron to another. This electrical synapse allows the rapid transfer of information. With chemical synapses, the presynaptic neuron releases neurotransmitters from storage vesicles into the synaptic cleft. These neurotransmitters bind to the postsynaptic neuron, causing either an excitatory or inhibitory reaction in the cell.[10][11][12]
Related Testing
The functionality of peripheral nerve fibers is testable in clinical practice, most commonly through nerve conduction studies (NCS) and electromyograms (EMG). An NCS is performed by placing a stimulating electrode along a peripheral nerve and recorded by a recording electrode on a muscle for a motor nerve study. The stimulating electrode may be placed on the skin with the recording electrode placed proximally on the nerve for sensory nerve studies. The electrical impulse gets generated by one electrode and recorded by the other. This impulse can generate different parameters of the study, including the amplitude, duration, and conduction velocity of the action potential. Some special tests, such as the F and H responses, can provide information about more proximal segments of the nerve. Information about peripheral nerve dysfunction can be collected from these studies. Indications for NCS include neuropathies, demyelinating conditions such as Guillain-Barre syndrome, and radiculopathies.[13][14]
During a needle EMG, the tester places needle electrodes through the skin directly into a muscle. As the patient contracts the muscle with increasing force, more motor units get recruited. Based on Henneman’s size principle, small, slow-twitch motor units are recruited at a lower threshold than large, fast-twitch motor units. One sign of nerve damage is when successive motor units are not recruited under normally sufficient muscle tension. This test is particularly helpful in diagnosing muscular conditions and those affecting the motor neuron unit and neuromuscular junction, such as muscular dystrophy, myasthenia gravis, and motor neuron loss in amyotrophic lateral sclerosis (ALS). In practice, neurologists often perform nerve conduction studies and electromyograms together.[15]
Pathophysiology
There are many etiologies in which nerve function can be abnormal with a wide range of consequences. PNS demyelination can occur from autoimmune processes, resulting in chronic inflammatory demyelinating polyneuropathy (CIDP) or acute demyelinating polyneuropathy in Guillain-Barre syndrome. CNS demyelination results in multiple sclerosis. Nerve damage from persistently elevated blood glucose results in diabetic neuropathy. Neuron degeneration and synapse dysfunction cause ALS, Parkinson disease, myasthenia gravis, Lambert-Eaton syndrome, and many other neurological conditions.[1][11]
Clinical Significance
Understanding nerve physiology is an essential step in the understanding of different types of peripheral neuropathy. The two main types of peripheral neuropathies are axonal and demyelinating neuropathies. The primary pathology in axonal neuropathy is an axonal loss, which usually starts distally in a dying back phenomenon and clinically presents with a length-dependent peripheral neuropathy. The common causes are those related to metabolic disturbances of the nerves such as diabetes, nutritional deficiencies, and organ failures. These neuropathies also affect more predominantly the smaller nerve fibers, which serve the sensory functions of pain and temperature. They are also frequently called small fiber peripheral neuropathy. The other primary type is demyelinating neuropathy, such as acute Guillain Barre syndrome and chronic inflammatory demyelinating polyneuropathy. Because the pathology involves predominantly the myelin sheaths, the larger and more proximal nerves will have involvement. As a result, these neuropathies are not length-dependent and involve both proximal and distal segments of nerves, including nerve roots. Clinically, the larger fibers are involved early, resulting in early loss of joint position and vibratory sensation and early loss of deep tendon reflexes.
Neurophysiologically, with nerve conduction studies, the axonal neuropathy is characterized by loss of nerve action potential amplitude, whereas demyelinating neuropathy is characterized by an early decrease in nerve conduction velocities.
The physiology of nerve impulses generation and conduction, how it is attenuated by myelin or the lack thereof, and intraneuronal communication have major clinical implications in the body. A firm understanding of these processes assists in interpreting conduction studies, making diagnoses, and effectively treating neurological conditions.