Continuing Education Activity
The middle cerebral artery (MCA) is the most common artery involved in acute stroke. It branches directly from the internal carotid artery and consists of four main branches, M1, M2, M3, and M4. These vessels provide blood supply to parts of the frontal, temporal, and parietal lobes of the brain, as well as deeper structures including the caudate, internal capsule, and thalamus. This activity describes the presentation, evaluation, and management of middle cerebral artery strokes, and explains the role of the members of the interprofessional team in assessing, diagnosing, managing, and rehabilitating patients who suffer from this, and how to try to prevent a recurrence.
Objectives:
Identify the various potential etiologies of middle cerebral artery stroke.
Summarize the most important evaluations needed to diagnose a middle cerebral artery stroke.
Outline the differential diagnoses that must be considered if a patient presents with stroke-like symptoms but does not have a stroke.
Review how to address the modifiable risk factors for secondary stroke prevention after a stroke, and how the interprofessional team can be involved in improving the outcome and quality of life of a stroke patient.
Introduction
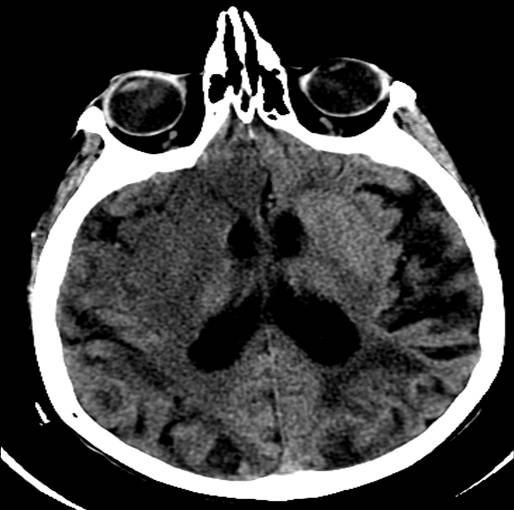
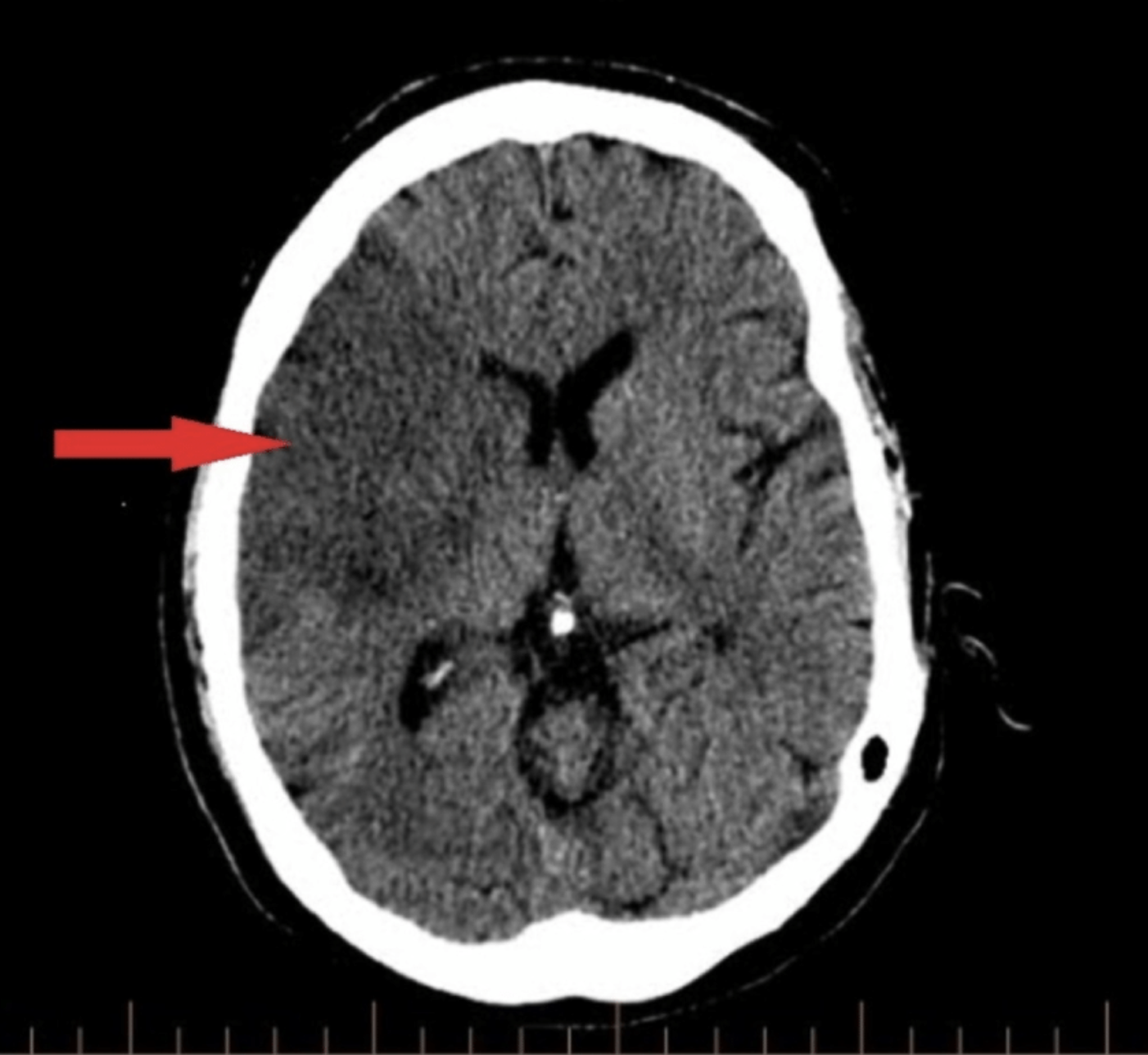
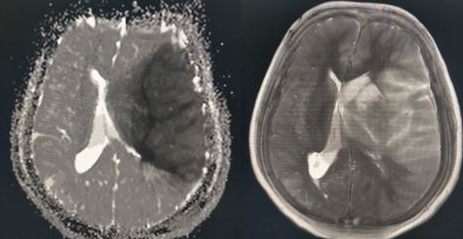
The middle cerebral artery (MCA) is the most common artery involved in acute stroke. It branches directly from the internal carotid artery and consists of four main branches: M1, M2, M3, and M4. These vessels provide blood supply to parts of the frontal, temporal, and parietal lobes of the brain, as well as deeper structures, including the caudate, internal capsule, and thalamus. See Images. Left Middle Cerebral Artery Territory Infarction, Right Middle Cerebral Artery Stroke. Its vast supply means that strokes involving the MCA territory can have a multitude of presenting symptoms, depending on which branches and structures are affected.[1] See Image. Anatomy of Brain Vascular Territories.
Etiology
There are multiple risk factors for strokes, which can be divided into modifiable and nonmodifiable categories—many of the causes of hemorrhagic and ischemic stroke overlap. The nonmodifiable risk factors of each include age, sex, race, and genetics. The risk increases as you get older; the risk is higher in men at a younger age, but the risk of death is higher overall in women; and the risk is significantly greater in African Americans, and slightly higher in Hispanics and Native Americans as well, compared to whites.
Modifiable risk factors, including hypertension, smoking, obesity, alcohol consumption, and diet all contribute to both ischemic and hemorrhagic stroke. However, hyperlipidemia, physical inactivity, diabetes, and cardiac causes such as cardiomyopathy, heart failure, and atrial fibrillation, are risk factors for ischemic stroke but not hemorrhagic stroke.
The etiology of hemorrhagic stroke is most frequently hypertension, especially in developing countries where the burden of hypertension is unknown due to infrequent screening and diagnosis. Less frequently, hemorrhagic strokes can also be due to angiopathies, which can be diagnosed with a cerebral angiogram.
Ischemic stroke is frequently broken into several etiological categories, including atherosclerotic, cardioembolic, lacunar, and cryptogenic. However, ischemic stroke can also, at times, be due to more specific causes such as vasculitis, dissections, or genetic disorders. In the case of atherosclerotic disease, severe stenosis or thrombosis can be caused by cholesterol plaques, which can cause occlusions of vasculature or stenosis of vessels, blocking blood flow and leading to cerebral ischemia. Cardioembolic strokes can occur in the case of atrial fibrillation or, in younger individuals, a patent foramen ovale, particularly in the setting of deep vein thromboses. In both of these cases, clots can travel from the heart through the left ventricle into the aorta and lodge in the internal carotid artery (ICA) or its branches, most frequently the MCA. The smaller deeper vessels that are the culprits of lacunar infarcts, such as the lenticulostriate arteries, are more often affected by hypertension and diabetes than by thromboses.[2]
Epidemiology
Stroke is the fifth leading cause of death in the United States and the second leading cause of death worldwide. The overall prevalence of stroke in the U.S. is 2.6% in adults over 20 years old. Approximately 85% of these are ischemic strokes, and over half of all ischemic strokes occur in MCA territory.[3] The risk of stroke is higher in men than women when young and middle-aged, but overall, women have a higher risk of stroke than men over the course of a lifetime, with the risk for women being 20% to 21% versus 14% to 17% in men. The risk of stroke is also higher in Blacks and Hispanics than Whites. Over the last few decades, stroke occurrence and mortality have overall decreased in the United States and other high-income countries, but no change in frequency has been observed in middle or low-income countries. In addition, mortality due to stroke has increased in middle and low-income countries. This is likely related to the advancements made in secondary stroke prevention in higher-income countries.[4]
History and Physical
When patients present with under 24 hours of neurological symptoms, such as weakness, dizziness, numbness, issues with speech, or visual changes, they are managed in the form of a stroke alert or a code stroke. This is done because there is a specific protocol that has to be followed to quickly obtain the most important components of the history, perform the pertinent parts of the physical exam, and get the emergent laboratory studies and relevant imaging. The most important component to obtain in the history of these patients is their last known normal, which is when they were last seen or last felt that they were at their baseline. The reason this is so important is that it determines what options are available for their management. If their last known normal is within 4.5 hours of presentation, they are within the window for receiving IV tissue plasminogen activator (TPA); if it is within 24 hours, they are within the window for neurosurgical intervention. Other additional important components of the history include when the symptoms started, what they were, and if they have changed, improved, or worsened since their onset. Whether or not the patient has any contraindications to TPA also needs to be obtained quickly. This includes any history of intracranial hemorrhage, ischemic stroke within the past 3 months, any recent invasive surgical procedures or recent neurosurgical or spinal surgeries, a recent history of any kind of internal bleeding, use of anticoagulant medications if they have had recent blood draws or IV lines at noncompressible sites, and recent trauma or myocardial infarction. Other components of history that are useful to know are the patient's medical problems, as issues such as diabetes, hypertension, and hyperlipidemia all increase the risk of stroke.[5]
The most important part of the physical exam to perform in an emergent fashion when there is a concern for a stroke is the National Institute of Health Stroke Scale. This scale is a standardized way of assessing stroke patients to remove subjectivity during their examination. This scale is particularly useful for identifying and localizing strokes involving the anterior circulation, such as the middle cerebral artery, based on the functions they assess. The components include sensation, strength, and coordination in all 4 extremities; production and comprehension of speech, including naming and repetition; visual fields; orientation to self and time; and symmetry and sensation of the face. Large MCA strokes are usually the easiest to recognize of all strokes, as they tend to present with the major deficits that one thinks of when thinking of a stroke, such as unilateral flaccidity, forced gaze deviation, visual field cuts, and, if in the dominant hemisphere, speech deficits. This scale is useful in predicting whether there will be findings on the diffusion-weighted MRI. When performing this exam, it is also important to note whether any abnormalities appreciated are chronic, such as residual deficits from previous strokes. In addition, it is necessary to check vital signs, particularly the patient's blood pressure as hypertension and hypotension can both be associated with neurological symptoms, and blood pressure must be under 180/110 prior to administration of IV TPA.[6]
Evaluation
There are two options for radiologic imaging in the setting of acute stroke - CT or MRI (see Image. Head CT, Right Middle Cerebral Artery Stroke).[7] Regardless of the route taken, it is necessary to obtain imaging without contrast as well as vascular imaging. The CT without contrast will assess for subacute to chronic strokes, any kind of hemorrhage, and hypodense signs that may indicate a large acute stroke. The MRI, particularly the DWI, will demonstrate the same things, as well as an acute stroke within minutes to hours. The next step is either a CT or MR angiogram of the head and neck with perfusion that images from the aorta up to the brain. The purpose of the angiogram is to assess for areas of stenosis or occlusions that may explain the symptoms. The purpose of the perfusion scan is to determine the extent of tissue that has already been damaged versus the extent that is at risk of damage, the core versus the penumbra. This determines whether or not the patient is a candidate for mechanical thrombectomy. The reason the neck is imaged is to include the internal carotid arteries down to the aorta to determine if these structures are involved. If the patient gets a CT and CT angiogram, they will eventually need an MRI as well.[8]
There are several important laboratory studies to be performed. One is a coagulation panel, particularly in the setting of a patient on warfarin, as it is important to know the patient's INR and whether or not it was at a therapeutic level. Complete blood counts and basic metabolic panels should also be sent. A point of care blood glucose should be obtained on initial patient evaluation to assess if this may be contributing to the patient's symptoms. Hypoglycemia can mimic stroke and can be life-threatening if not recognized and treated promptly. More laboratory studies that need to be evaluated in patients with stroke are glycosylated hemoglobin levels and a lipid panel. This is because part of secondary stroke prevention involves optimizing these levels, which will be discussed later. Lastly, cardiac enzymes should be sent as well to assess for cardiac abnormalities.[5]
After a stroke has been identified, the patient typically will need to get an EKG and a transthoracic echocardiogram, as well as telemetry monitoring, in order to determine whether there is an intracardiac thrombus or cardiac abnormality that may be the source of the patient's stroke. Some of these abnormalities that increase the risk of stroke include atrial fibrillation, a patent foramen ovale, or akinesis or hypokinesis of the cardiac walls. Assessment for a patent foramen ovale is typically indicated in patients of a young age or with recurrent strokes of unknown etiology. If a cardioembolic source is likely and these studies are all normal, the patient will likely get a transesophageal echocardiogram or cardiac MRI to get a better visualization of the left ventricle for a thrombus and the left atrial appendage for a thrombus, respectively. If these are still negative, the patient will likely get a Holter monitor on discharge, typically for 30 days, to see if any arrhythmia is captured.[9]
Treatment / Management
In the acute setting, the interventions for an MCA stroke are IV tissue plasminogen activator (TPA) and thrombectomy, if the patient qualifies. As mentioned above, the time of onset will determine if the patient qualifies for these interventions. For IV TPA, the last known normal has to be within 4.5 hours of the administration of the thrombolytic. For thrombectomy, the last known normal has to be within 24 hours of intervention.[10] Outside of this window, there is no abortive therapy, and further treatment is geared toward symptom management and secondary stroke prevention.[5] Symptoms that may need to be managed in the acute setting vary with the severity of the stroke and will be further discussed in the section addressing complications.
Secondary stroke prevention is aimed at modifiable risk factors, which were briefly addressed in the section on etiology. Modifiable risk factors include diabetes, hypertension, hyperlipidemia, and smoking. The first value that is addressed is glycosylated hemoglobin, which is used to measure the presence and severity of diabetes. In a stroke patient, the goal is less than 6.5%. This needs to be managed with appropriate diabetic medications, dietary modification, and close monitoring of blood glucose as well as frequent checks of glycosylated hemoglobin levels in those above or approaching this value. This should be checked approximately every three months.
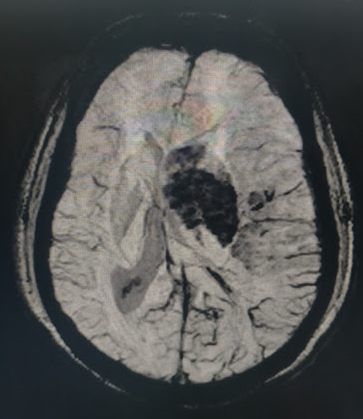
Blood pressure is another important modifiable risk factor. In the first 24 hours after the last known well, there is a period of permissive hypertension to prevent further ischemic injury to tissue at risk in the setting of low cerebral perfusion pressure. If the patient does not receive IV TPA, the goal is less than 220/120 mmHg. If the patient does receive TPA, it is less than 185/105 mmHg in order to decrease the risk of hemorrhagic transformation (see Image. Hemorrhagic Transformation Within the Left Middle Cerebral Artery Territory Infarction). After these 24 hours, the goal drops to normotensive, less than 140/90 mmHg, for the patient’s lifetime. It is important to adjust blood pressure medications accordingly, and for the patient to closely monitor their blood pressure at home.
In order to manage hyperlipidemia, the patient needs their lipid profile checked, the target of which is the level of low-density lipoprotein (LDL). The goal LDL in a patient who has had a stroke is less than 70. If it is not at goal, they need a high-intensity statin medication, typically atorvastatin or rosuvastatin. If they are already on this medication before the stroke, the dosage needs to be increased if they are not at goal. If they are already at the maximum dose, this needs to be continued as such, regardless of whether they are at goal or not. LDL needs to be closely monitored every few months, particularly if the patient is newly started on a statin or their dosage is changed, in order to monitor whether their cholesterol levels are responding appropriately.
Another important modifiable risk factor is smoking. It is necessary to emphasize the importance of smoking cessation to a patient who is an active smoker and has had a stroke.[7]
The final piece in secondary stroke prevention, and possibly the most important, is addressing the need for an antiplatelet or anticoagulant. The appropriate medication varies with the presentation, previous medication, and stroke etiology. If the patient does not receive TPA and has an NIHSS score of less than 4, it is recommended to use dual antiplatelet therapy with aspirin, between 81 mg to 325 mg at the discretion of the physician, and clopidogrel 75 mg daily for 3 months. After these 3 months, the patient can be continued on monotherapy. If the NIHSS score is greater than 4, the recommendation is to load with aspirin. If they were already on aspirin 81 mg daily, their dosage might be increased to 325 mg, or they may be switched to clopidogrel 75mg daily.[11]
If they are found to have an intracardiac thrombus or atrial fibrillation, or another reason for anticoagulation, such as a recent history of deep venous thrombosis (DVT), then they can be continued on anticoagulation, and there is no indication from a neurologic perspective for antiplatelets. Studies have indicated that there is no significant difference in stroke recurrence in the setting of holding anticoagulation versus resuming within 24 hours (48 if the patient receives IV TPA). However, some studies have indicated a slightly increased risk for hemorrhagic transformation if it is resumed in 48 hours up to 7 days. Typically, due to this difference in the risk of hemorrhagic transformation, it is recommended to hold anticoagulation in the immediate period following acute ischemic stroke.[12][13]
Differential Diagnosis
The differential diagnosis in a patient with stroke-like symptoms is extensive because there are a large number of stroke mimickers. A stroke must be ruled out in the acute setting as its management is highly time-sensitive. Once a stroke is ruled out, many other differentials must be considered. As described previously, MCA strokes typically present with the symptoms individuals associate most commonly with strokes, such as unilateral weakness and/or numbness, facial droop, and speech deficits ranging from mild dysarthria and mild aphasia to global aphasia.
Some dangerous central processes can be mistaken for stroke, such as subdural hematomas (SDH), intracranial hemorrhages (ICH), or masses, which also need to be recognized quickly. Masses, ICH, and SDH can cause stroke-like symptoms based on location or mass effect on the same structures in the brain that are supplied by the MCA. These may qualify for urgent surgical interventions, so it is important to identify these early. They would likely be diagnosed based on the CT or MRI that is performed in the acute setting when evaluating for stroke.
Seizures, particularly status epilepticus, can mimic stroke due to gaze deviation and loss of movement, as well as global aphasia due to loss of consciousness. This is important to diagnose acutely as well as it requires urgent treatment with benzodiazepines and antiepileptic drugs.
Demyelinating disorders such as multiple sclerosis may also be confused with stroke given presentations consistent with vision loss and numbness or weakness that typically will favor a particular extremity. This is something that will require an MRI with and without contrast to differentiate, as well as a thorough history and clarification of symptom onset, as it usually is not as acute and likely waxes and wanes.
Toxic, infectious, and metabolic differentials must be included as well, which is why several laboratory studies must be sent in the evaluation of acute stroke. Sepsis, uremia, hypo and hyperglycemia, hyponatremia, and hyperkalemia can all cause symptoms that resemble a stroke. Encephalopathic presentations due to these abnormalities and others can mimic stroke in that they can cause aphasia and apparent deficits in strength, which is why the assessment of laterality is so important in recognizing stroke versus some of its mimics. In these presentations, it can be more challenging to localize a possible central nervous system lesion as the deficits are usually more generalized. In these situations, in addition to the labs that are sent in the acute setting, there will need to be a more extensive laboratory workup, including an infectious workup.
Complex migraines can mimic stroke as well, as some migraines may present with unilateral weakness or numbness. This is something that imaging cannot help diagnose. Typically there will be a headache associated with the symptoms, and the patient will likely have a history of migraines and similar symptoms in the past. This is something that requires a thorough history to diagnose. Usually, treating the headache will result in symptom resolution.
Lastly, some psychiatric disorders may present with stroke-like symptoms, such as unilateral numbness or weakness. Examples of these are conversion disorder and panic attacks. These should be lower on the differential diagnosis, as other mimics need to be urgently diagnosed and treated, and can be dangerous and even fatal if misdiagnosed. These are typically diagnoses of exclusion and must be diagnosed based on a carefully obtained history.[14]
Prognosis
The prognosis of middle cerebral artery strokes depends on several factors. The most essential factors in determining prognosis are the size of the stroke, whether the patient received thrombolytic therapy and/or thrombectomy, and access to rehabilitation following the stroke. Explaining the prognosis after a stroke to the patients and their families can be challenging, particularly in the acute setting. It can take from weeks up to a year to reach a new baseline level of function. Patients who suffered smaller cortical strokes typically recover rapidly within a few weeks and then begin to level out over a few months. However, for larger strokes, it can be challenging to give a prognosis in even the first three months because the process of recovery varies so drastically between individuals. Mental status by day four usually can give the prognosis of the patient's mental status moving forward, but activities of daily living may take up to six months to establish a new baseline. Of note, physical therapy is as important in older patients as it is in younger patients in improving function.[15]
In the case of severe MCA strokes, in which patients often battle cerebral edema and alteration of consciousness, mortality is frequently dependent upon whether life-saving measures are taken. Many patients qualify for tracheostomy and percutaneous gastrostomy tube placement, which increases the length of hospital stay and also increases the risk of infection. However, if they do not receive these interventions, their life expectancy is markedly decreased due to the inability to protect their airway or consume nutrients. In these patients, the prognosis is grim at best, the likelihood of recovery is very low, and their length of time of survival depends on whether or not these interventions are performed.[16]
Complications
There is an extensive number of complications that can occur following MCA stroke. Most stroke patients will develop some type of complication at some point during their recovery. According to one study of over 300 patients, 85% experienced at least one complication during their hospital stay alone, and most of the remaining individuals developed at least one complication within 6 weeks following their stroke. The patients were then followed over 30 months. The most common complications to occur in stroke patients while they are still hospitalized include infections, particularly pneumonia or urinary tract infections; falls; and pain. Less common complications include pressure sores, seizures, recurrent stroke, thromboembolism, and psychological complications, particularly depression. Following discharge and in the 30 months over which the patients were followed for this study, the most common complications continued to be infections, falls, and pain, but depression and anxiety also rose in prevalence. Concern for seizures and recurrent stroke persisted, and hospital readmission joined the list, while thromboembolism was only seen in one case.
The explanation as to why falls, pain, and infections are the most common complications has to do with the functions that MCA strokes impact most. Mobility is affected, particularly in the setting of decreased sensation and strength, which makes falls more likely to occur. Complications such as dysphagia can lead to aspiration, while decreased mobility can contribute to atelectasis, and both of these can promote the development of pneumonia. This decreased mobility is a large contributing factor to pressure sores and pain as well. This is why interdisciplinary teams are crucial in the recovery and improvement of quality of life of stroke patients.[17]
Patients who suffer more severe strokes tend to suffer more dangerous and potentially fatal complications. These include cerebral edema, depression of consciousness, severe dysphagia, and inability to protect their airway. When cerebral edema occurs, which typically peaks around day 3 to 5 following acute stroke, this can put pressure on the ventricles and cause midline shift. If the patient does not have significant atrophy or just has very significant swelling, this can quickly progress to herniation and death. If it does not get to this point, it can still lead to severe depression of mental status, which can cause patients to be unable to protect their airway or swallow safely. This then leads to the difficult question of whether the patients should get tracheostomies or percutaneous gastric tubes placed, and these themselves can then lead to further complications and infections, while not getting these performed can lead to fatal outcomes as described previously in the section on prognosis.[16]
Deterrence and Patient Education
The primary education that is given to patients regarding strokes is risk factor modification for secondary stroke prevention and the warning signals for stroke, at which time they should call 911 and go to the nearest stroke center.
Secondary stroke prevention is implemented by addressing modifiable stroke risk factors, which include hypertension, hyperlipidemia, diabetes, diet, obesity, and smoking. Smoking cessation counseling is an important component of education, as is counseling regarding lifestyle and dietary modifications. Patients are typically started on aspirin if they had not been on it previously. Their cholesterol and glycosylated hemoglobin levels are closely monitored every few months, and if the patients have hyperlipidemia or diabetes, these are addressed with the appropriate medications. Patients are also advised to closely monitor their blood pressure with an at-home blood pressure cuff, and antihypertensive medications are adjusted accordingly.[7]
As for alarm signs, the acronym FAST has been used for years, which is recently being replaced with BE FAST, which stands for balance, eyes, face, arms, speech, and time. This can be elaborated upon so that patients have a better picture of alarm symptoms and when to return to the emergency department. Balance indicates falling in one direction or dizziness; eyes include sudden unilateral loss of vision or sudden onset double vision; face indicates facial drooping; arms stand for arm weakness or loss of sensation; speech can have multiple meanings including slurring, word-finding difficulty, or difficulty getting words out; and time indicates that "time is brain" and the faster they get to an emergency department, the better.[18]
Enhancing Healthcare Team Outcomes
Without an interdisciplinary team involved in the care of a stroke patient, many aspects of care and needs of the patient and family may slip through the cracks. In addition, and most importantly, the team members, typically including stroke neurologists, nurses, care coordinators, physical, occupational, kinesiological, and speech therapists, and pharmacists, each address different concerns and needs, and all make important contributions when it comes to discharge planning and the future management of the patient after their hospitalization.
It is important to have stroke specialists on the team, which include a stroke neurologist, but also needs to include nurses and therapists who have been trained to care for stroke patients. This is important, so the patient gets the care that is tailored to them and their needs. Pharmacists are often on the teams as well. They are helpful when it comes to medication reconciliation, education of physicians and nurses regarding medication management, as well as education of the patient and their families. Pharmacists are also needed to assess what needs the patients will have on discharge, such as medication teaching or dose packs and pill containers. Other important members of the team, depending on the patient's functional status, may include chaplains, palliative care, and neuropsychologists. Therapists are necessary to assess and recommend needs on discharge, such as inpatient rehabilitation and homecare. The interdisciplinary team and their daily or bi-weekly rounds are important for the appropriate care and planning for patients. These individuals are important as each of them can address different needs of the patients, and are all involved in the inpatient care, including medications, treatments, assessment, therapies, and family education, as well as discharge planning, such as the best way to provide medications based on insurance, rehabilitation and home care needs, appropriate medications and physician follow up, and patient and family education.[7] Studies have demonstrated that a team-based approach with multiple phases including discussion of a plan, formulation of a written plan, and implementation of this plan via conferences and video chats between physicians, nurses, therapists, care coordinators, and administrators results in improved function without a change in hospital length of stay or rate of discharge. This improvement in function, which is the primary endpoint for stroke patients, supports the importance of communication between healthcare workers in making plans for their patients to manage them in the best way possible and provide the greatest opportunity for an improved quality of life.[19]