Continuing Education Activity
Gangrene is tissue damage secondary to infection, ischemia, or both. It is a relatively uncommon condition, and early recognition is essential. Gangrene is associated with a high incidence of mortality, and in patients that survive, it can have a massive impact on the quality of life. This activity reviews the identification, evaluation, and treatment of gangrene, highlighting the role of the interprofessional team in managing patients with this condition.
Objectives:
Identify the etiology for the development of gangrene.
Describe the appropriate assessment for a patient with gangrene.
Explain the management strategies of gangrene.
Review the importance of an interprofessional approach to the evaluation and treatment for patients with gangrene.
Introduction
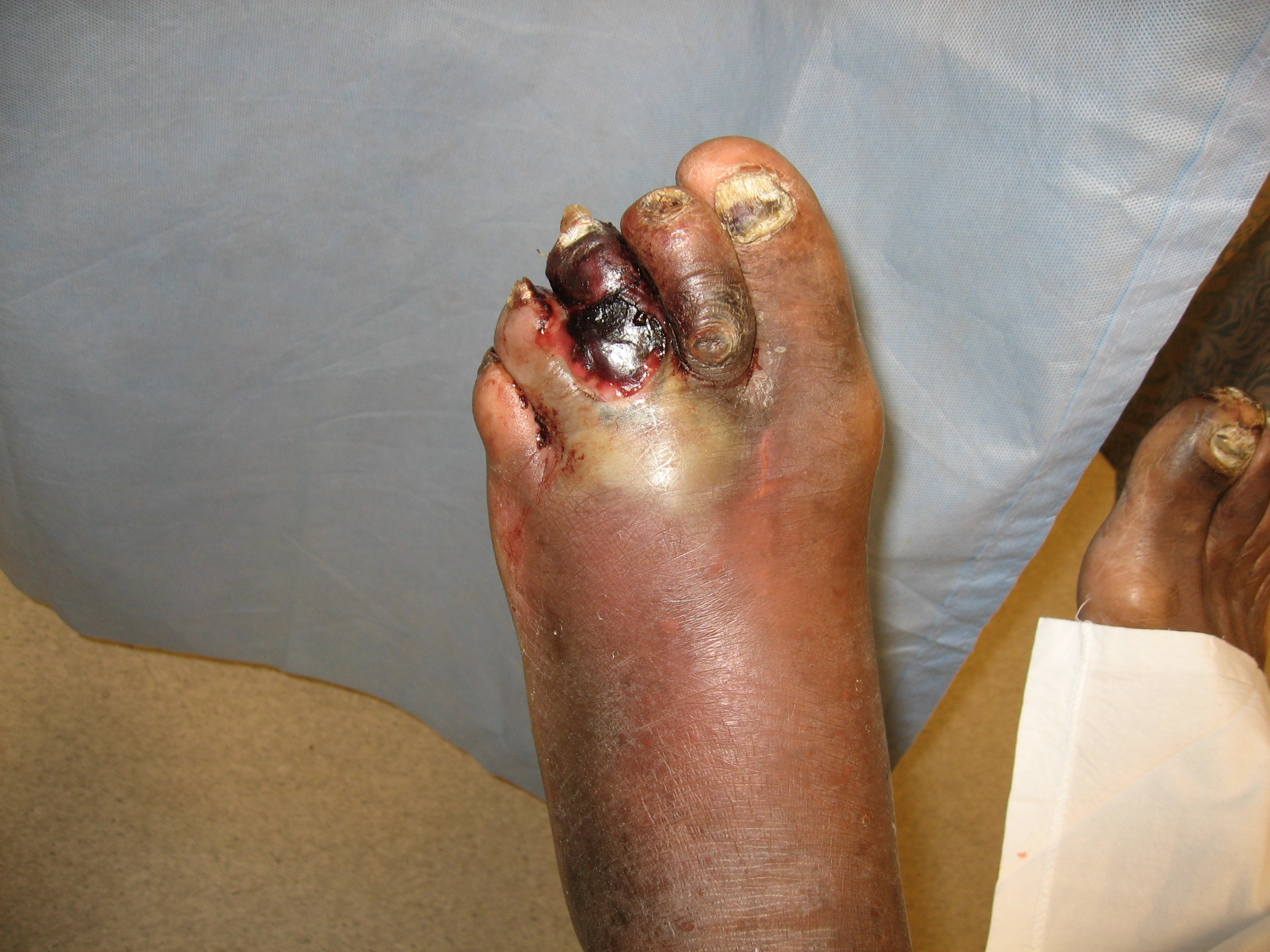
Gangrene is a clinical condition of ischemic and necrotic tissue, often circumferential around a digit or extremity. It is identified by discolored or black tissue and associated sloughing of natural tissue planes. The three main types of gangrene are wet gangrene, dry gangrene, and gas gangrene.
Dry gangrene is dehydrated ischemic tissue caused by progressive ischemia distal to arterial occlusion, often a progression of peripheral artery disease. Wet gangrene, which may be dry, complicated by a secondary infection, has associated edema and erythema but no crepitus. Gas gangrene is a specific type of necrotizing infection with edema, crepitus, and gas on radiographs. Necrotizing soft tissue infections overlap with the infectious causes of gangrene and involve necrotic skin lesions that may extend into subcutaneous, fascial, and muscle compartments.[1][2]
The associated tissue loss in gangrene can significantly decrease life quality due to associated pain, limited mobility, and increased risk of hospitalization. These conditions can also progress to substantial morbidity and mortality, with the risk of multiple surgeries and death with the disease progression.[3]
Etiology
Dry/ischemic gangrene is most commonly secondary to atherosclerosis and progressive occlusion of the peripheral arterial blood supply to distal tissue. The risk factors of peripheral atherosclerosis overlap with the risk factors for coronary artery disease: diabetes, smoking, hypertension, and hyperlipidemia. Conditions that increase blood demand, such as localized infection and trauma, may worsen limb ischemia.[4] Dry gangrene is often aseptic as bacteria fail to survive in the dry and mummified tissue.[5]
Less common causes of ischemic gangrene are vascular occlusions from other pathology. Thromboembolic disease may rarely result in the cessation of arterial flow if thrombosis is transferred downstream, and arterial thromboses may develop in situ in a hypercoagulable state. Trauma to the limb or vascular system may result in ischemia and gangrene. Vasculitis, adventitial cystic disease, popliteal entrapment, and Buerger disease may also contribute to gangrene development.[4][6] These conditions may also result in acute limb ischemia, which can progress to gangrene if severe. Acute limb ischemia is defined by a sudden decrease in limb perfusion, diagnosed within two weeks of symptom onset. The symptoms of acute limb ischemia are classically identified by the 6 Ps mnemonic: paresthesia, pain, pallor, pulselessness, poikilothermia (disorder temperature regulation), and paralysis.[7]
Ischemic limb gangrene may also occur in limbs with intact peripheral pulses due to thromboses in the microcirculation. Venous limb gangrene is one possible cause where micro thrombosis happens in the same limb as an acute large-vein thrombosis, typically in a hypercoagulable state. Symmetric peripheral gangrene is another condition where multiple limbs may develop symmetric gangrene despite adequate perfusion; an example is purpura fulminans in patients with septicemia secondary to Neisseria meningitidis.[7]
Wet gangrene occurs when tissue compromised by poor venous or arterial blood flow becomes infected. This is most commonly seen in areas prone to edema (lower extremities/feet), though it also can be seen in genitourinary and oral tissues. Diabetic patients are more susceptible to these infections due to poor wound healing and hyperglycemia.[5]
Gas gangrene is historically caused by infection with Clostridium perfringens and other Clostridium species (C.septidum), resulting in clostridial myonecrosis. This organism can cause rapid development of localized tissue necrosis and systemic signs of illness in part due to its production of exotoxins and is characterized by the presence of gas in subcutaneous tissue. Additional bacterial infections may also result in gas production and the rapid spread of infection, including Escherichia coli, Bacteroides, Staphylococcus epidermidis, and streptococcal infections.[8] Type I necrotizing fasciitis, characterized by friable superficial fascia, dishwater-gray exudate, and an absence of pus, is another bacterial infection caused by a polymicrobial mix of aerobic and anaerobic organisms that may also cause gas in tissue.[9]
Epidemiology
Ischemic/dry gangrene occurs as tissue loss most commonly seen with progressive peripheral artery disease (PAD). Critical limb ischemia/chronic limb-threatening ischemia (CLI/CLTI) is the most advanced stage of peripheral artery disease, with an incidence of 1% of the United States population over 50 years old and up to twice that over 70 years old.[4] Lower extremity peripheral artery disease itself affects more than 200 million people worldwide, and up to 10% of people with PAD have CLI/CLTI. Over five years, between 5-10% of patients with asymptomatic PAD or minimal symptoms with intermittent claudication, may progress to CLI/CLTI.[6]
Gas gangrene typically occurs after trauma, with anaerobic bacteria's introduction into a previously protected tissue space. Gas gangrene has been identified after traffic accidents, crush injuries, gunshot wounds, and postoperative complications related to infection. Non-traumatic gas gangrene has also been documented from hematogenous spread, and multiple case studies have demonstrated an association with a metastatic gut malignancy.[10] Similarly, other necrotizing soft tissue infections typically have a defined entry point due to trauma or postoperative surgical site complications.[9] Gas gangrene is relatively rare, with approximately 1000 cases per year in the United States; 50% are attributed to traumatic injuries, 30% to postoperative complications, and 20% as a spontaneous infection.[11]
Pathophysiology
In ischemic gangrene, reduced arterial perfusion leads to arteriole dilation as compensation, resulting in distal edema and endothelial damage. This can trigger a cycle of micro thrombosis resulting in worsening tissue damage. Due to the ischemic environment, localized cellular dysregulation limits the ability to have adequate wound healing and set the tissue up for continued damage and infection.[6]
In gas gangrene, bacteria such as C.perfringens and group A streptococcus can produce multiple exotoxins, resulting in local tissue destruction and subsequent systemic infection.[10] Alpha-toxin, a C-lecithinase, can result in extensive tissue necrosis and promote systemic hemolysis.[11]
History and Physical
Patients with critical limb ischemia/chronic limb-threatening ischemia (CLI/CLTI) at risk for developing gangrene will present with limb pain that has progressed from intermittent claudication with exertion to chronic rest pain. The pain may be worsened in an elevated leg and improved in the dependent position due to compromised blood flow. If a concurrent neuropathy is present (most commonly from diabetes), then there may not be a consistent history of pain, and tissue loss may be the first presentation of ischemia.[4]
On physical exam, patients with ischemic gangrene will have rubor of the affected extremity when dependent, and early pallor with elevation. Capillary refill will be reduced, and there may be a loss of overlying hair. Ankle pulses are frequently absent. The location of tissue loss may help identify the cause; ischemic tissue loss typically involves the toes and distal foot; venous ulcerations often overlie the malleoli; neuropathic ulcerations usually start on areas of pressure along with the sole.[4][6] A complete exam of the affected extremity should include identification of any neuropathy and a probe-to-bone test in the setting of any ulceration or tissue loss to identify the depth of tissue injury and risk of osteomyelitis.[12]
Wet gangrene should be suspected if there is associated drainage and edema in the setting of a patient with a previous foot ulcer or tissue injury secondary to diabetes or ischemia. Additionally, plantar tenderness increases the concern for a possible deep foot abscess and should be evaluated thoroughly.[3]
Severe acute limb ischemia will present with significant lower extremity pain, sensory loss (ranging from mild at the toes to permanent diffuse nerve damage), and paralysis of the affected limb. The arterial flow will be inaudible initially, followed by inaudible venous flow as the disease process progresses.[7]
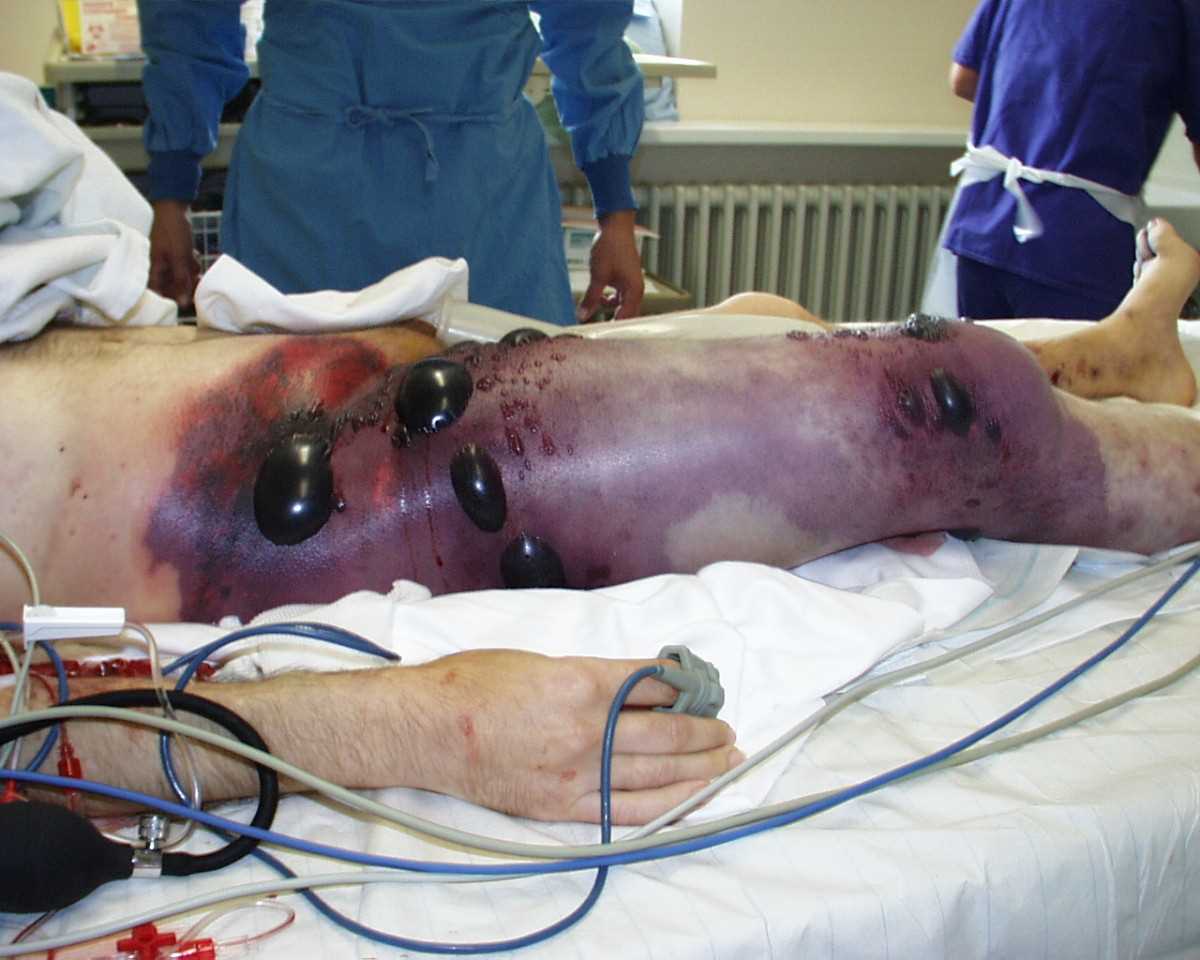
Gas gangrene presents with pain, edema, the development of hemorrhagic bullae, and color changes ranging from a pale coloration to a bronze-purplish red coloration. The patient often has an associated history of trauma or recent surgical intervention.[5] Necrotizing soft tissue infections, particularly those caused by Streptococcus pyogenes (group A streptococcus), may start as an erythematous initial lesion and progress to a dusky skin coloration with overlying hemorrhagic bullae within 24 to 72 hours of injury. Once the skin becomes gangrenous and starts sloughing, there is a high mortality risk. Patients with necrotizing infections of any type often present with edema, fever, malaise, and pain out of proportion to exam findings – this subtle presentation increases the risk of delayed diagnosis, and high clinical suspicion is essential.[9]
Evaluation
The laboratory evaluation of ischemic gangrene is focused on identifying clinical risk factors such as renal failure, hyperlipidemia, and diabetes, which would impact the treatment plan. Assessments for concurrent infection are also appropriate, though wound cultures are less helpful without additional signs of infection such as localized erythema and swelling.[4][12]
Other tests recommended for ischemic gangrene are focused on identifying the arterial disease's level and complexity, which will help narrow down treatment options. Non-invasive testing like an ankle-brachial index (ABI) is essential for early identification of PAD in a patient with tissue loss and is considered abnormal if less than 1.0. Ankle pressure below 40-60 mmHg is also consistent with critical ischemia, and in the presence of tissue loss, <70 mmHg is considered abnormal.[13][4] If the ABI is consistent with arterial disease, then additional imaging is used to localize the lesion(s) – Duplex ultrasound, digital subtraction angiography, CT angiography, and MRA are all options.[4] If the ABI is not conclusive, which is common in diabetic patients and those with advanced age as vessel compressibility may be affected, additional non-invasive testing can include ankle pressures, toe pressures, and transcutaneous oxygen pressures.[6]
Multiple classification systems exist for the staging of critical limb ischemia/chronic limb-threatening ischemia (CLI/CLTI). The recently developed WIfI (wound, ischemia, foot Infection) system by the Society of Vascular Surgery uses a combination of wound classification, ischemia severity, and the presence of foot infections to provide prognostic guidance regarding treatment recommendations and the anticipated response to revascularization.[4] The specific wound portion of the WIfI classification is rated between 0-3, with 1 being minor tissue loss that may be salvageable with simple amputation, 2 is often gangrene limited to digits (and therefore treatable with multiple digital amputations or transmetatarsal amputation), 3 is the most severe with extensive tissue loss, requiring amputation proximal to the transmetatarsal level for treatment. Using the classifications of WIfI, patients with chronic limb ischemia can be clinically staged by the risk of amputation. They can be classified based on the anticipated benefit of revascularization as well.[13]
If gas or wet gangrene is suspected, Gram stain and wound cultures can help identify the bacterial cause to guide antibiotic therapy, but the diagnosis is typically made clinically. Surface wound swabs are rarely helpful due to potential contamination of skin bacteria, and the sample should be obtained from deep swabbing or aspiration of purulent discharge.[14]
Additionally, X-rays may be able to identify subcutaneous gas, which is always pathologic, and found with gas gangrene and type I necrotizing skin infections.[5][9] CT, with contrast demonstrating a lack of fascial enhancement, and MRI with abnormal signal intensity in the deep fascia, can also help establish the diagnosis. However, surgical intervention should not be delayed if there is clinical suspicion. If the diagnosis is unclear, patients can be evaluated by local exploration under bedside local anesthesia; the return of 'dishwater' fluid and easy dissection of fascial planes is characteristic of necrotizing infections and can be followed up by definitive surgical treatment.[15]
Treatment / Management
Treatment of ischemic gangrene is focused on restoring blood flow to help reduce rest pain and heal ischemic wounds. Once ulcers have progressed to dry gangrene, it is unlikely that the tissue will recover completely, but tissue loss can be minimized by medical and surgical management.[4][5] Medical treatment of ischemic gangrene includes the use of antiplatelet therapy with aspirin or clopidogrel and treatment of hypertension with beta-blockers and angiotensin-converting enzyme inhibitors. Hyperlipidemia should be treated with a statin as appropriate, and patients with diabetes should achieve adequate glucose control, ideally to a hemoglobin A1C less than 7%. Smoking cessation is vital for reducing the risk of disease progression.[4][6][5][12]
Surgical treatment of limb ischemia is focused on revascularization to improve pain and prevent limb loss. In the setting of acute ischemia, catheter-based intravascular thrombolysis can be used. Otherwise, revascularization can be pursued with endovascular intervention with balloon angioplasty (with or without stent), and surgical therapy can bypass a stenotic area or directly remove a blockage. The decision to pursue bypass or endovascular treatment is dependent on the lesion and the patient’s comorbidities, and early involvement in a multi-disciplinary vascular team, if available, is recommended.[4][6]
Primary amputation (amputation before an attempt at revascularization) is recommended if there is significant necrosis of the weight-bearing portion of the foot, refractory pain, sepsis/uncontrolled infection, paresis of the extremity, or limited life expectancy.[4][12] Often, above-ankle amputation is recommended if there is extensive foot necrosis.[6][3] Autoamputation is also a possibility, the spontaneous separation of the unviable tissue from the viable tissue; however, a case series of patients with diabetes-related dry gangrene found that only 1 of 12 developed autoamputation – all others required surgical amputation.[2] If more than two digital ray amputations are required to treat necrosis, it is recommended to consider transmetatarsal amputation of the forefoot instead to preserve function – multiple toe amputations can adversely affect pressure distributions and result in worsening pressure injuries.[12]
Treatment with hyperbaric oxygen therapy has been proposed as a method to increase oxygen tension in ischemic tissue. There has not been a demonstrated benefit with critical limb ischemia (CLI). Other potential experimental treatments include the use of growth factors and stem cell therapy to promote angiogenesis; however, there is a lack of clinical data. This treatment is currently limited to clinical trials.[6]
If an infection is suspected as wet gangrene, either based on systemic signs of infection, localized erythema/drainage, or plantar foot pain, urgent surgical drainage and debridement (possibly with minor amputation) are indicated. Antibiotic treatment should be started for all patients with suspected chronic limb-threatening ischemia (CLTI) and additional deep foot infection or wet gangrene. The appropriate dressing should be used to retain moisture without adding maceration.[12][3] Empiric antibiotic choice should depend on patient risk factors and local susceptibility rates; gram-positive coverage is usually indicated, with broadened coverage in diabetic patients to include potential MRSA or gram-negative coverage.[14]
Gas gangrene, with associated exotoxins, can be very aggressive with a high mortality rate when treatment is delayed. Therefore surgical exploration and debridement are recommended as soon as possible to gauge the extent of infection and obtain specimens for culture/Gram stain. The initial surgical site often will need to be re-evaluated and debrided multiple times. Surgery within 24 hours of admission is associated with increased survival.[9][15] Fasciotomies will also help to decompress fascial compartments and promote blood flow. Gangrene of the trunk cannot be amputated, so aggressive debridement is required.[11]
Antibiotic treatment of gas gangrene and necrotizing soft tissue infections should be tailored to the causative organism as soon as possible; however, in the immediate initial evaluation, broad-spectrum treatment with coverage for gram-positive, gram-negative, and anaerobic bacteria should be used. If group A streptococci or Clostridium are identified, the recommended treatment is penicillin with clindamycin for 10 to 14 days; clindamycin is especially recommended to reduce toxin production monotherapy with clindamycin is not recommended due to increased inducible resistance rates. Patients with necrotizing infections are often systemically ill and should be managed based on sepsis guidelines for fluid resuscitation and treatment of associated organ damage.[9]
Differential Diagnosis
Gangrene is typically fairly unique with visible necrotic tissue. The differential diagnosis of limb pain can include
- Diabetic neuropathy
- Complex regional pain syndrome,
- Nerve root compression
Other potential causes of local ischemia not listed above are
- Frostbite
- Ergotism (localized vasospasm with thrombosis)
- Compartment syndrome
- Calciphylaxis, a rare condition seen in renal failure patients.[16][17]
The differential diagnosis for gas gangrene include:
- Group A streptococcal infections
- Septic shock
- Toxic shock syndrome
- Abdominal abscess
- Vibrio infections
Prognosis
Within one year of the diagnosis of critical limb ischemia/chronic limb-threatening ischemia (CLI/CLTI), up to 40 to 50% of patients with diabetes will have an amputation, and 20 to 25% will die.[4] Additional observational studies observed an amputation rate of 19% at six months and 23% at 12 months in nondiabetic patients with rest pain and ischemic ulcers/gangrene. The most common indication for amputation was an untreatable infection.[13][18] Patients should be followed for at least two years after revascularization procedures to evaluate for any recurrence of CLTI.[12]
Gas gangrene has a significant fatality rate; up to 25% of trauma patients with gas gangrene die, with an increase to 100% if treatment is delayed or inadequate. Poor prognosis is associated with increased age and multiple underlying comorbidities and a location on the trunk.[11]
Complications
While most limb salvage treatment is focused on limiting amputations, especially major amputations (above the ankle, requiring a prosthetic for ambulation), amputation may also be optimal for some patients to allow for participation in rehabilitation with a prosthesis. However, retrospective studies have demonstrated that only 65% of patients with below-the-knee amputation and 29% of above-the-knee amputation amputees were ambulatory at one year.[19] Observational studies of patients two years after below-the-knee amputation demonstrated that 15% had a contralateral amputation, 15% progressed to an above-the-knee amputation, and 30% were dead.[17]
Consultations
All patients with suspected critical limb ischemia/chronic limb-threatening ischemia (CLI/CLTI) should be referred to a vascular specialist for consideration of limb salvage unless major amputation is urgently needed.[12]
Necrotizing soft tissue infections with wide or disfiguring debridements should engage multidisciplinary surgical teams as soon as possible; orthopedics, plastic surgery, urology, and colorectal diversion may be needed depending on the location and depth of the surgery.[15]
Deterrence and Patient Education
Patients should be educated on the proper foot and wound protection to promote healing and prevent a recurrence. This includes education on appropriate shoes and insoles, as well as early identification of signs of inflammation.[12] Patients should get medical attention immediately if they develop any symptoms of tenderness, redness, disproportionate pain, or fever. Educate intravenous drug users about potential fatal complications of gas gangrene due to the injection of contaminated heroin or other chemicals.[20]
Enhancing Healthcare Team Outcomes
The early identification of ischemic tissue, whether secondary to infection or peripheral vascular disease, is essential to the success of the treatment strategies listed above. A detailed history and physical, including ankle-brachial index in patients at risk for peripheral artery disease, can identify patients at risk before tissue loss develops.[17] (level of evidence 4) Once ischemia is detected, the use of validated classification scores can provide consistent communication across multiple treatment teams and the patient regarding the risk of amputation and the likelihood of improvement with revascularization.[13] [Level 2]
For necrotizing soft tissue infections and gas gangrene, worsening mortality has been identified with the delay in surgical treatment more than 24 hours after admission, so the entire clinical team is essential in considering the diagnosis and pursuing evaluation and treatment once identified to reduce patient mortality risk.[11] [Level 3]