Continuing Education Activity
Cutaneous melanoacanthoma is a benign epithelial lesion that typically presents as a solitary black plaque or nodule, usually on either the head, neck, or trunk of an older adult in their seventh decade. The clinical features of a cutaneous melanoacanthoma mimic those of melanoma. As a result, diagnosis can be challenging as clinical, dermoscopic, and microscopic evaluations may necessitate biopsy for confirmation. Treatment involves conservative excision, with close monitoring for recurrence. While melanoacanthoma can occur in the oral mucosa as a reactive lesion, the presentation differs from cutaneous melanoacanthoma. Syndromes like Laugier-Hunziker syndrome may present with metachronous oral melanoacanthomas.
This activity reviews the diagnosis, evaluation, and treatment of cutaneous melanoacanthoma and highlights the role of the interprofessional team in evaluating and treating patients with this condition. Given the diagnostic nuances of this benign lesion, interprofessional collaboration among primary care clinicians and dermatologists may enhance the quality of care, leading to reduced morbidity and mortality.
Objectives:
Identify the clinical presentation of cutaneous melanoacanthoma by physical examination, signs, and symptoms.
Determine the most definitive test to diagnose cutaneous melanoacanthoma, including imaging modalities or biopsies.
Interpret the histopathology findings of cutaneous melanoacanthoma with available evidence-based guidelines.
Collaborate with the interprofessional team to enhance care delivery for patients with cutaneous melanoacanthoma.
Introduction
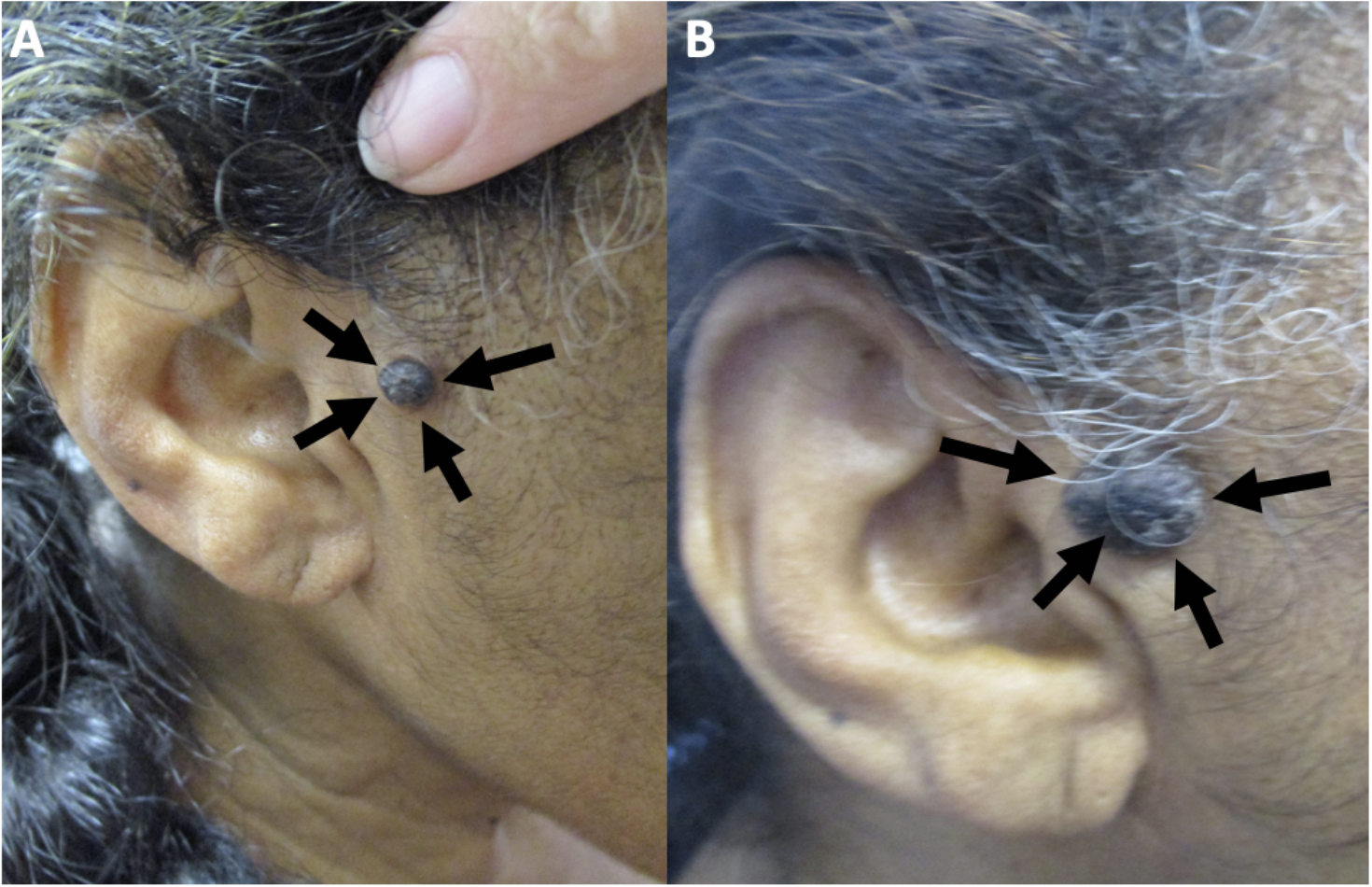
Melanoacanthoma is a rare, benign cutaneous neoplasm. The lesion is more common in older adults, with a median age of 65 and a male predominance. Cutaneous melanoacanthoma has an unclear etiology, though some argue the presentation is a heavily pigmented seborrheic keratosis variant.[1] The initial presentation is typically a solitary, slow-growing black nodule on the head, neck, trunk, or extremities (see Image. Cutaneous Melanoacanthoma on the Right Preauricular Cheek).[2] Clinical examination, dermoscopy, and reflectance confocal microscopy of melanoacanthoma may be equivocal compared with malignant disease, so a biopsy with histopathologic evaluation is essential.[3] Microscopic examination shows an epithelial lesion with hyperkeratosis, acanthosis, and papillomatosis. The epidermis has mixed melanocytes with large dendritic processes and keratinocytes. Further staining with Fontana-Masson, S100 protein, or Melan-A (ie, MART-1) may be required to pinpoint a diagnosis.[4] Conservative excision of cutaneous melanoacanthoma is the treatment of choice since incomplete removal of the tumor can result in persistence and continued growth or recurrence of the lesion. Follow-up for patients with cutaneous melanoacanthoma should include evaluating any new or recurring lesions.
Melanoacanthoma, called oral melanoacanthoma, may be present in the mouth (or other mucosal epithelium) and is thought to be reactive without tumor-like progression.[5] See the StatPearls companion resource, "Oral Melanoacanthoma," for more information.[6] In contrast, the neoplasm can also occur on non-mucosal sites as a benign neoplasm and is designated as a cutaneous melanoacanthoma.[7][8][9] Considerable debate exists if pigmented seborrheic keratosis and cutaneous melanoacanthoma are 2 distinct entities, but they are considered separate, given their unique clinical features and management.[10][11][12][13]
Melanoacanthoma is associated with other syndromes. Metachronous oral melanoacanthomas were found in patients with Laugier-Hunziker syndrome, and melanoacanthoma on the lower lip has been associated with plasma cell cheilitis and Langerhans cell hyperplasia of the lower lip.[14][15]
Etiology
The etiology of melanoacanthoma has not been definitively established, though some have proposed a reactive process.[15] The secondary colonization by dendritic melanocytes of nonmelanocytic lesions has been observed, a common feature in reactive processes (eg, lichen simplex chronicus, verrucae, squamous cell carcinoma). Dendritic melanocyte colonization may account for these pigmented cells found at all epidermal levels in a melanoacanthoma. The colonization may be observed in sites of trauma, which is postulated to lead to irritation, lesion formation, and rapid augmentation of the lesion.[10][16][17] Superficial changes, like ulceration, may occur related to poor blood flow, friction, pressure when lying down, and trauma.[12] However, although features are similar to reactive processes, cutaneous melanoacanthoma is a neoplasm, differing from oral melanoacanthoma, which is thought to be purely reactive. Therefore, some etiologic factors are unexplored.
Epidemiology
The results of studies are limited regarding melanoacanthoma to establish definitive epidemiologic trends. The incidence of melanoacanthoma has been shown to range from 1 in 100,000 pathology specimens to between approximately 1% and 38% of seborrheic keratoses. Studies vary on the incidence, from 0.8% to 38%. However, many of these results have varying amounts of inclusion of pigmented seborrheic keratosis; earlier evaluations of seborrheic keratoses may have neglected the diagnosis of cutaneous melanoacanthoma.
Melanoacanthoma appears in older adults (usually aged greater than 60 years) with a median age of 65 but can appear across all adults. Most cases have been reported in men, but no clear gender predominance is apparent. Of 52 melanoacanthomas in 1 study, 28 were in men. Melanoacanthoma occurs worldwide. Reports of patients originate from many countries, including Belgium, Brazil, Canada, India, Italy, Japan, South Korea, Spain, and the United States of America.[2][7][2][9][13][9][18] The patient’s race or nationality was described for 43 individuals. Melanoacanthoma has been most described in white patients, who comprise 42% of reported cases.[19][18][20] Other reported patients were Hispanic (8 patients), Indian (6 patients), Asian (4 patients: 3 Japanese and 1 Korean), Black (4 patients), Brazilian, Haitian-Creole, and Italian.[8][10][11][12][17][21][22][23]
Pathophysiology
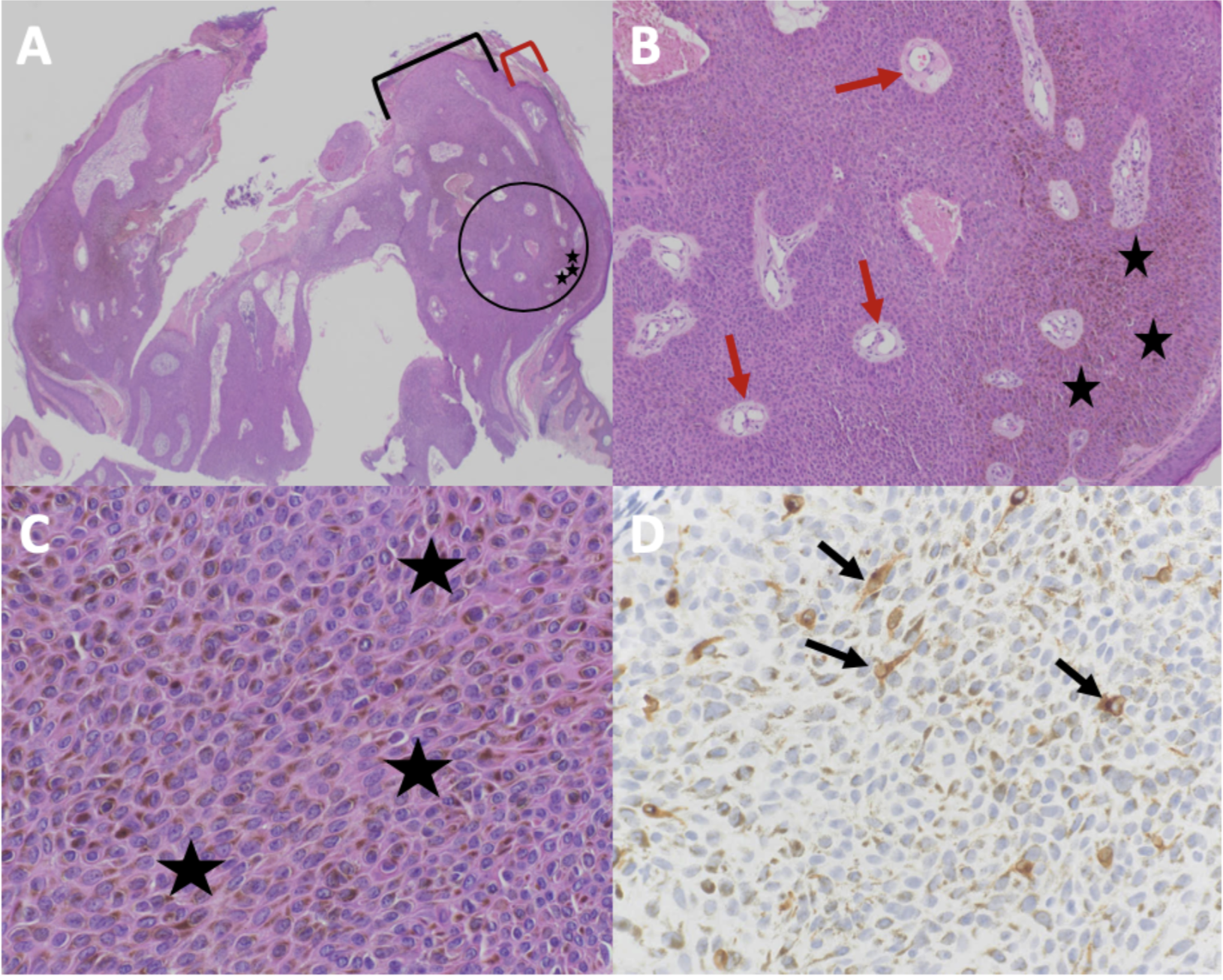
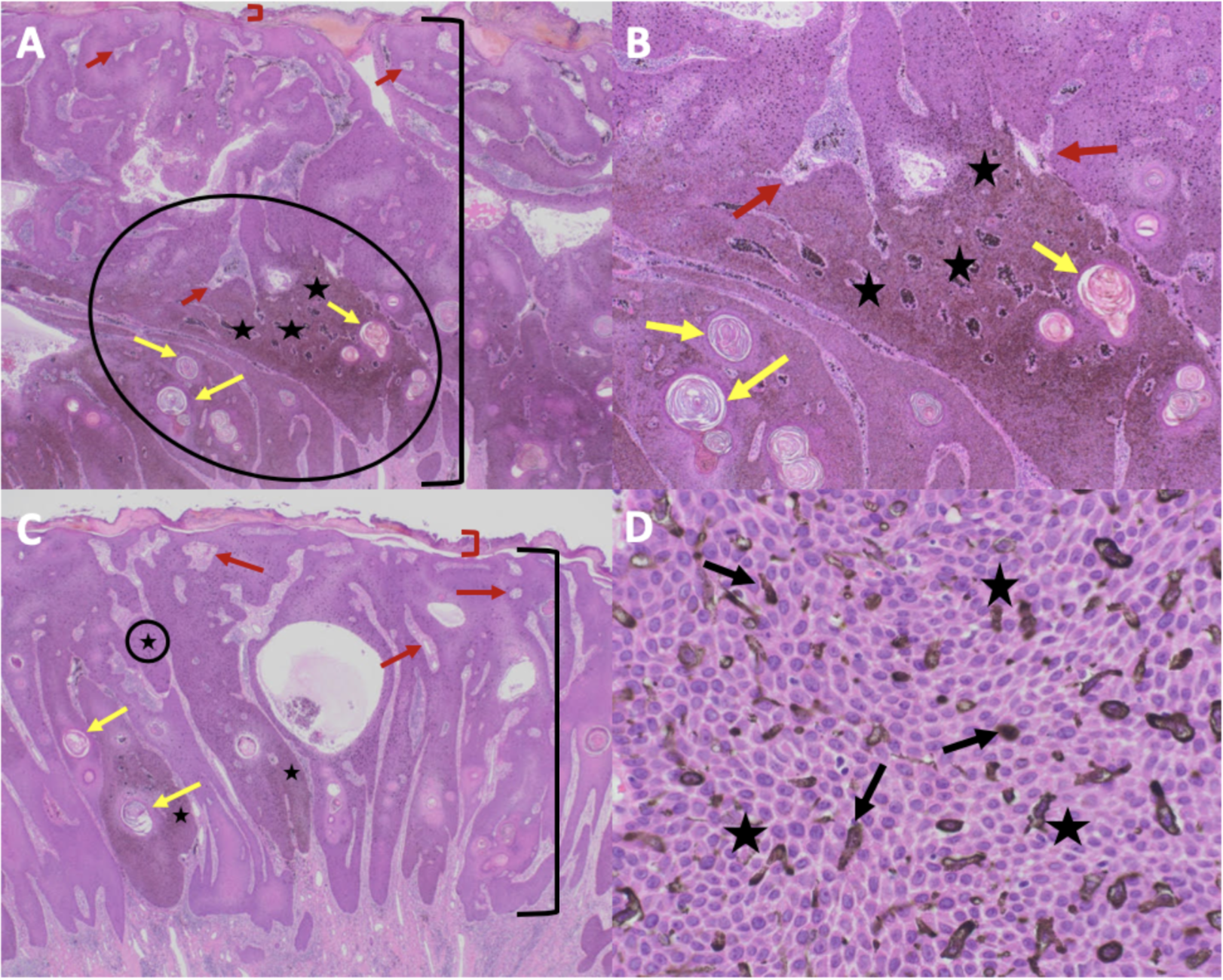
The pathophysiology of melanoacanthoma is unclear. Unlike seborrheic keratosis, trauma-induced cutaneous injury and partial inhibition of melanin pigment transfer from large melanocytes with numerous dendritic processes to adjacent keratinocytes may have a role in the pathophysiology of melanoacanthoma.[24] In 1 study, 20% to 50% croton oil placement or excision (to replicate trauma) was performed on half of a seborrheic keratosis to see if the formation of melanoacanthoma would occur. In contrast to melanoacanthomas, where the melanocytes are dispersed throughout the epidermis, the melanocytes of the irritated and traumatized seborrheic keratoses predominantly remained in the epidermal basal layers at the junction between the epidermis and dermis (see Image. Cutaneous Melanoacanthoma, Histopathology).[25] These findings implicate a pathogenesis different from seborrheic keratosis and more related to reactive or other pathogenesis.[21]
Histopathology
Microscopic features of an H&E-stained melanoacanthoma (see Image. Cutaneous Melanoacanthoma, Microscopic Features) include:
- Thickening of the stratum corneum (hyperkeratosis)
- Thickening of the entire epidermis (acanthosis)
- Absent cellular atypia of the keratinocytes
- Undulation of the epidermis (papillomatosis)
- Hyperpigmentation in all epidermis layers (correlating with melanocytes found in all epidermal layers)
- Keratin-filled pseudocysts in the epidermis
- Perivascular lymphocytic inflammation in the dermis [8][19][26][27][28]
Some researchers consider melanoacanthoma to have 2 histologic variants, either with melanocytes unevenly scattered throughout the epidermis or in nests with keratinocytes in the epidermis.[12][17] Stains may be required to pinpoint a diagnosis, including Fontana-Masson, S100 protein, or Melan-A (ie, MART-1). Combining these stains should differentiate the lesion from melanoma, which may appear similar clinically.[7][13] Because melanoacanthoma may have nests of melanocytes throughout the epidermis, careful evaluation for any atypical nests consistent with melanoma should be elucidated through stains, including cytokeratin stains (eg, cytokeratin 7), which may highlight keratinocytes to distinguish the lesion from melanoma.[18][23] If brown chromagen is used to identify the positive-staining cells when immunoperoxidase stains are performed, differentiating the brown-staining melanocytes from the brown-appearing melanin-laden keratinocytes is imperative. However, if red chromagen is used, this problem is resolved since only the melanocytes show the red staining.
Electron microscopy confirms the presence of highly dendritic melanocytes and reveals the defective transfer of melanin from these melanocytes to the adjacent keratinocytes. Also, Langerhans cells may be present throughout the epidermis except in the basal layer.[10]
History and Physical
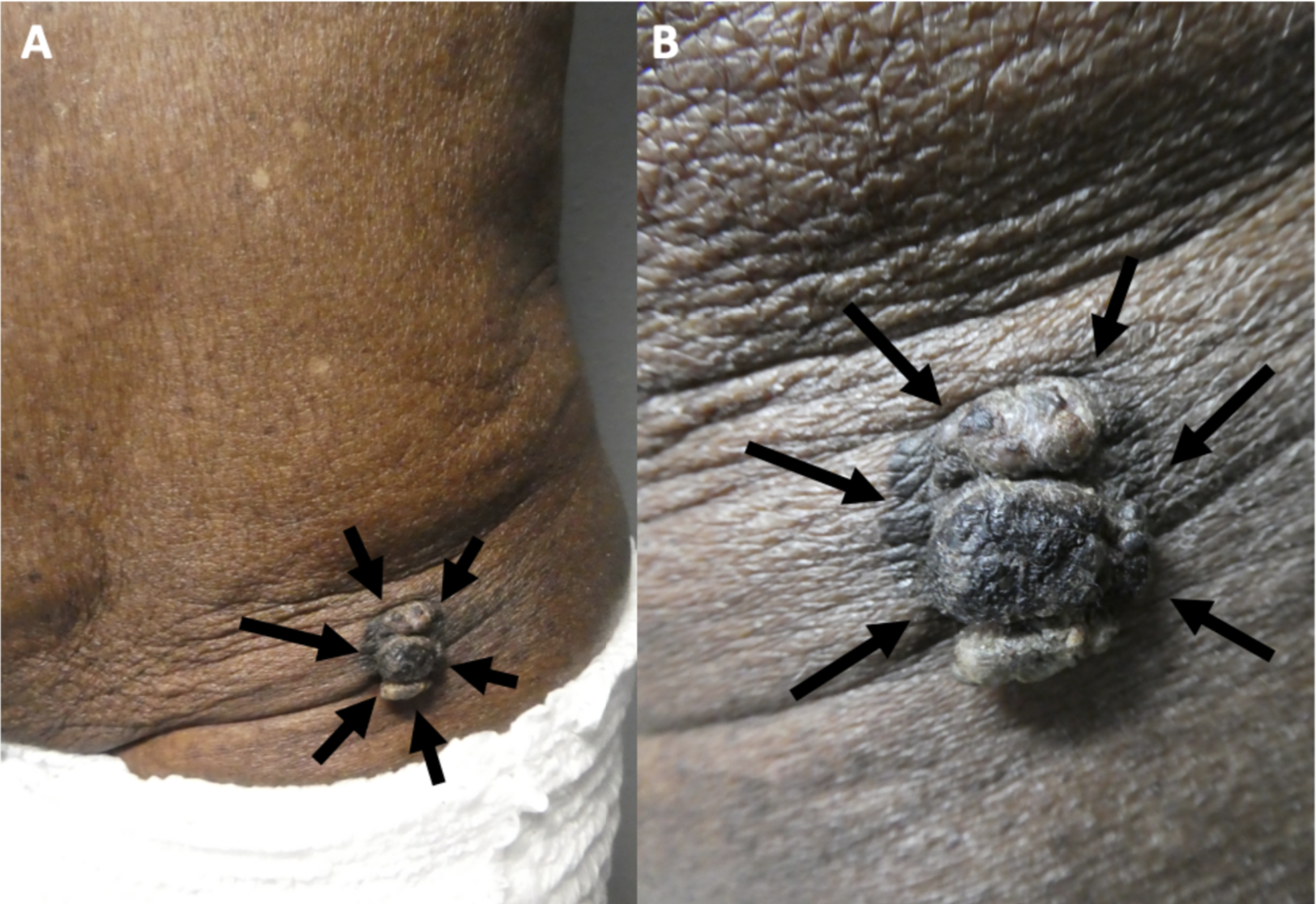
Melanoacanthoma is a solitary, pigmented, asymptomatic, slow-growing papule, nodule, or plaque. Although uncommon, they can grow rapidly, itch, be tender, or become irritated.[22] Melanoacanthomas may be present for weeks to years before diagnosis; they range from 0.2 to 15 cm in size, with a median size of 2 cm.[11][17][11][19] Most importantly, half of the lesions identified are black and may express multiple colors (eg, black and blue, black and gray, black and brown), which resembles melanoma.[23][29] Occasionally, the surface of a melanoacanthoma may be lobulated or verrucous.[8][10][18][10][8][21][20] Usually, melanoacanthoma presents on the head, neck, or trunk, though some have been reported on extremities, genitals, or buttocks. Multiple lesions are rare, unlike oral melanoacanthomas.[5] Rarely, a melanoacanthoma presents as a cutaneous horn or exophytic, mimicking verrucae or a squamous cell carcinoma (see Image. Cutaneous Melanoacanthoma, Left Lower Abdomen).[12]
Evaluation
A partial or excisional biopsy is the most appropriate approach for evaluating a lesion when diagnosing melanoacanthoma. An adequate biopsy specimen should be obtained so that the pathologist has sufficient tissue to examine and thereby be able to provide an accurate diagnosis.[3] Other tools that may show promise include dermoscopy and reflectance confocal microscopy, though these alone, based on the available literature, are insufficient to diagnose the condition.
Dermoscopy
Dermoscopic features of melanoacanthoma vary from mimicking malignant lesions to benign lesions. The results of some studies show that the dermoscopy of melanoacanthoma is similar to a pigmented Spitz nevus, including a starburst pattern consisting of symmetrically distributed pigmented streaks on the periphery. This pattern has been considered diagnostic for a pigmented Spitz nevus. Features of melanoma may be visualized when performing a dermoscopy of melanoacanthoma, including gray to whitish hyperkeratotic areas, multiple black-to-brown dots, multiple globules, blue-white veil, polymorphous vessels, and granularity. Dermoscopy may visualize benign lesions, with a regular pigmentary network showing a cribriform pattern of ridges and fissures, comedo-like openings, hairpin vessels, milia-like cysts, moth-eaten border, and sharp demarcations.[11][20]
Reflectance Confocal Microscopy
Reflectance confocal microscopy of melanoacanthoma has been reviewed in multiple studies. Findings included keratotic debris-filled surface depressions and ridges at the level of the stratum corneum of the epidermis, which correlates with the verrucous lesion. Numerous tangled bright dendritic cells may be noted in the suprabasal spinous and granular layers of the hyperplastic epidermis.[18] At the spinous-granular layer, a typical honeycomb pattern may be present with epidermal projections, keratin-filled invaginations, and widespread dendritic pagetoid cells. Dendritic cell infiltration can occur at the dermal-epidermal junction between the dermal papillae. Melanophages may be noted at the level of the papillary dermis.[22]
Treatment / Management
Sometimes confused for melanoma on visual examination, most melanoacanthomas are traditionally surgically removed either at the time of biopsy or subsequently by simple excision. Partial removal during the biopsy of a melanoacanthoma has resulted in the persistence of the preauricular lesion in at least 1 patient.[21] Also, the recurrence of a nasal melanoacanthoma was observed 6 months after removal in one reported case.[10] Other therapeutic interventional modalities may be considered, including cryotherapy with liquid nitrogen or topical 5-fluorouracil 5% cream.[11][12]
Differential Diagnosis
While benign, melanoacanthomas have a wide differential that should include:
- Actinic keratosis: Pigmented actinic keratoses should show atypia during the evaluation of a skin biopsy specimen.
- Basal cell carcinoma: Pigmented basal cell carcinoma may present similar to melanoacanthoma but should be distinguished by histopathology.
- Melanocytic nevus: If the lesion is raised, distinguishing melanoacanthoma without histopathologic evaluation is challenging. However, some melanocytic nevi are flat and can be distinguished clinically from melanoacanthoma.
- Melanoma: Clinically, melanoma (including lentigo maligna) appears nearly identical to melanoacanthoma. However, if an H&E-stained specimen is nondiagnostic, histopathologic evaluation with stains may need to be used to distinguish the 2 entities.
- Pigmented spindle cell (Spitz) nevus
- Seborrheic keratosis: In seborrheic keratosis, the pigment and melanocytes are predominantly present and restricted to the basal or lower epidermis layers. In contrast, in a melanoacanthoma, the pigment results from the dendritic melanocytes that are present throughout not only the lower layers but also the upper layers of the epidermis.
- Solar lentigo: Solar lentigo is usually flat, while melanoacanthoma is generally raised.
- Squamous cell carcinoma: The absence of cellular atypia and numerous mitoses excludes squamous cell carcinoma.
- Verruca vulgaris: The absence of koilocytes or koilocytic change excludes the diagnosis of verrucae.[2][19][22]
Prognosis
The prognosis for an individual with melanoacanthoma is excellent, as the lesion is benign and not associated with systemic manifestations. Follow-up of lesion excision usually reveals no recurrence, though scarring and other sequelae of excision may be present.[17][23] Some lesions may persist or recur, but this is less likely; lesions are unlikely to progress to malignancy.[10][21]
Complications
Melanoacanthoma is a benign epithelial lesion without any observation of malignant transformation. However, the lesion may occur adjacent to a malignant lesion, which may obfuscate diagnosis. Although most melanoacanthomas grow slowly, they can become giant, measuring over 10 cm in greatest diameter. Also, albeit less common, some melanoacanthomas have been noted to grow rapidly. In either of these settings, the lesion can become irritated and tender from rubbing against the adjacent skin or the overlying clothing. Rarely, a larger melanoacanthoma may spontaneously ulcerate or superficially infect.[12]
Deterrence and Patient Education
Melanoacanthomas are benign but mimic other benign and malignant lesions, like seborrheic keratosis or melanoma, respectively. Therefore, older adults with lesions resembling cutaneous melanoacanthoma must seek appropriate evaluation. Noninvasive examination methods, including clinical inspection, dermoscopy, and reflectance confocal microscopy, can be performed but are insufficient to confirm a diagnosis. Therefore, when a melanoacanthoma is possible, an appropriate lesion biopsy should be performed to confirm the diagnosis and exclude other conditions.
Pearls and Other Issues
Melanoacanthoma is a rare benign epidermis lesion; however, the actual incidence remains to be established. The clinician who evaluates the patient seldom considers melanoacanthoma. Most often, when a biopsy specimen is submitted, either melanoma or seborrheic keratosis or both are suggested. Complete removal of the melanoacanthoma is recommended since the lesion may persist following partial removal.
Enhancing Healthcare Team Outcomes
The clinical morphology of a melanoacanthoma can mimic that of another benign skin tumor, a seborrheic keratosis. However, for many patients in whom this epithelial neoplasm has been described, the initial impression was a melanoma. Therefore, the patient’s primary clinician should consider performing a complete skin check on the patient. Detecting a new or progressively enlarging asymptomatic black lesion should prompt the primary clinician to refer the patient to a dermatologist or surgeon for evaluation, including a lesion biopsy. Periodic follow-up by the clinician to confirm healing and ensure no persistence or recurrence of the melanoacanthoma is recommended.