Introduction
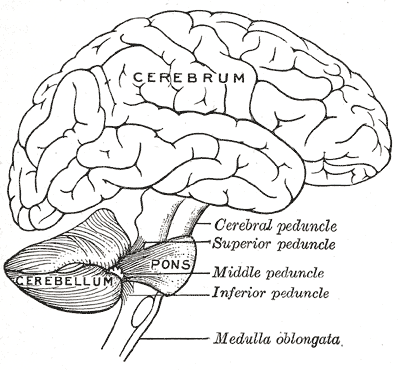
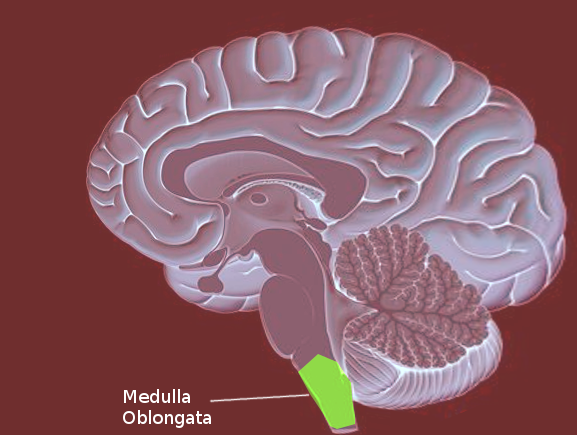
The medulla oblongata is the connection between the brainstem and the spinal cord, carrying multiple important functional centers. It is comprised of the cardiovascular-respiratory regulation system, descending motor tracts, ascending sensory tracts, and origin of cranial nerves IX, X, XI, and XII. Motor neurons cross from the left motor cortex to the right side of the spinal cord in the medulla. The medulla is the most caudal aspect of the brainstem, approximately at the level of the foramen magnum. Anterior to the medulla oblongata is the median fissure, which connects with the median fissure of the spinal cord. The posterior surface of the medulla can divide into two parts, the inferior part, which has median sulcus continuous with the spinal cord, and the superior part, which forms the lower floor of the fourth ventricle. The medulla, including the pons and the midbrain, is divided into three laminae, from dorsal to ventral, called the tectum, tegmentum, and basis, respectively. The tectum of the medulla involves the inferior medullary velum, which is the most inferior posterior part of the fourth ventricle. The tegmentum consists of the inferior olivary nucleus and the cranial nerve nuclei of IX, X, XI, XII. The basis, most ventral layer, has the pyramid decussation at the medulla.[1]
Structure and Function
The medulla includes multiple nuclei and tracts that have information from the spinal cord as well as the higher cortex. Each nucleus in the medulla or tract passing through will have a separate explanation below.
The cardiovascular-respiratory function of the medulla: Multiple studies show that the cardiovascular system and the respiratory system unite as one regulating system within a specific part of the medulla. The rostral ventral lateral medulla (RVLM) has been found to have the excitatory neurons that carry information to the pre-sympathetic neurons in the spinal cord, which maintain baseline arterial pressure. Within the RVLM, there is also the ventral respiratory column, which is known to be the center of control of respiratory rhythm and pattern. The ventral respiratory column divides into multiple sub-nuclei that establish connections with the presynaptic fibers in the RVLM and regulate the oscillating respiratory pattern that allows us to perfuse our tissues with oxygen. The caudal ventrolateral medulla contains synaptic input from tonic inhibitory baroreflex control. Together both the caudal and the rostral part of the ventral medulla have been known to be an essential location for input and convergence for controlling respiration with cardiovascular regulation.[2]
The Nucleus of the Solitary Tract (NTS): The NTS is in the dorsolateral medulla, from the caudal part of the facial nucleus to the caudal aspect of the pyramidal decussation. The organization of this nucleus is by the type of information transmitted, and the pathways needed to be activated. It is essential for analyzing and coordinating visceral afferent information. The afferent cardiorespiratory signals from peripheral chemoreceptors, pulmonary stretch receptors, and baroreceptors, synapse in the caudal and intermediate sections of the NTS, before reaching the RVLM in the medulla. They work in the regulation of respiration. Neurons carrying taste sensation also synapse in the NTS first before traveling to the thalamus and later on to the cortex.[3]
Area Postrema: This structure lies on the dorsal surface of the medulla at the floor of the 4th ventricle, rostral to the obex, adjacent to the solitary nucleus. It is also known as the vomiting center. It is positioned to detect emetic toxins in the blood and the CSF. The cells in the area postrema are unique because they don’t have a blood-brain barrier to keep out large polar molecules. These cells receive innervation via vagal afferents leading to the solitary nucleus, which is one way a person can feel nauseous and vomit. Studies have shown a lesion in the AP does not entirely inhibit vomiting from all of the emetic drugs. Vomiting from motion sickness is also reported to be not associated with the area postrema.[4][5]
Spinal trigeminal Nucleus: This brainstem nucleus is in the lateral medulla. It further subdivides into the trigeminal nucleus pars oralis, pars interpolaris, and pars caudalis, named rostral to caudal, respectively. The caudal portion predominantly receives nociceptive afferents from the face. The other nuclei incorporate sensory information from the sensory branches of the trigeminal cranial nerve (V) of the ipsilateral side of the face. The sensation of temperature, pain, and a deep or crude touch of the ipsilateral face are sent to this nucleus as well. It is the first central synapse and relay in the orofacial pain nerve fibers, which later reach the ventral posteromedial nucleus of the thalamus.[6][7]
Inferior Olivary nuclei: The inferior olivary nuclei are in the superior medulla. They are two structures lateral to the pyramidal columns in a C shape, composed of grey matter. It is associated with the inferior cerebellar peduncles (ICP), and many neurons travel through the ICP to the cerebellar cortex. The inferior olivary nuclei receive information from multiple sources carrying proprioception, muscle tension, and motor intention. The neurons from this nuclei are important because they synapse directly on the Purkinje cell bodies in the cerebellum. Due to the critical connection with the cerebellum, atrophy of the inferior olivary nuclei will lead to a cerebellar loss as well.[8]
Reticular formation: This is a net-like system found in the tegmentum of the midbrain, pons, and medulla, as well as the subthalamus and thalamus. This system is a complex organization divided into four regions. The 4th zone is the only one in the medulla between the medial and lateral columns. This zone plays a role in autonomic regulation of respiration, heart rate, and blood pressure.[9]
Pyramidal decussation of the motor pathway: This is the most ventral and most caudal part of the medulla, which is where the majority of the motor fibers from the motor cortex in the cerebrum decussate in the medulla and form the lateral corticospinal tract in the spinal cord. Some of the fibers that don’t cross the midline become the anterior corticospinal tract.
The Cuneate Nucleus and Gracilias Nucleus: These nuclei are most dorsal and caudal in medulla on the same level as the pyramidal decussation. The dorsal column- medial lemniscus pathway synapse into second-order neurons from their perspective dorsal root ganglia neurons at these nuclei. This pathway conveys conscious proprioception, fine tactile discrimination, and vibration sensations from the body. The cuneate nucleus, lateral to the gracile nucleus, receives sensory information from the upper extremities. The gracile nucleus receives sensory input from the lower extremities. Both nuclei travel cephalad and from the medial lemniscus once they decussate in the medulla.
Medial Leminscus: The neurons that form the medial lemniscus are the internal arcuate fibers from the cuneate and gracile nucleus in the caudal medulla. Once they decussate, they form the medial lemniscus pathway and terminate in the ventral posterior nucleus of the thalamus, carrying proprioception, vibration, and fine touch modalities. The medial lemniscus pathway is located in between the two inferior olivary nuclei and is dorsal to the pyramidal motor pathway. It travels through the rest of the medulla up to the level of the pons and midbrain.[10]
Spinothalamic tract: The spinothalamic tract includes the anterior and lateral tracts. Pain and temperature sensation travel through the lateral tract, and crude touch is through the anterior tract. The second-order neurons reaching the medulla from the spinal cord have already crossed over through the anterior white commissure in the spinal cord segment. In the medulla, the spinothalamic tract is between the inferior olivary nucleus and the spinal trigeminal nucleus. This tract goes through the whole brain stem terminating in the ventral posterior lateral nucleus in the thalamus.[11]
Embryology
The brain develops from the anterior terminus of the neural tube. In early development, the neural tube expands into three primary vesicles at four weeks, forebrain (prosencephalon), midbrain (mesencephalon) and hindbrain (rhombencephalon). Ultimately, the rhombencephalon divides into two secondary vesicles at five weeks, the metencephalon and the myelencephalon. The myelencephalon constitutes of the medulla oblongata and the lower part of the fourth ventricle.
Blood Supply and Lymphatics
The blood supply to the medulla can divide into two groups, which are the paramedian bulbar and lateral bulbar arteries. The paramedian bulbar arteries arise from the vertebral arteries and supply the medial aspect of the medulla. At the most caudal part of the medulla, the paramedian bulbar arteries can also arise from the anterior spinal artery. The lateral bulbar branches arise from the vertebral artery or the posterior inferior cerebellar artery and supply the lateral part of the medulla.
Nerves
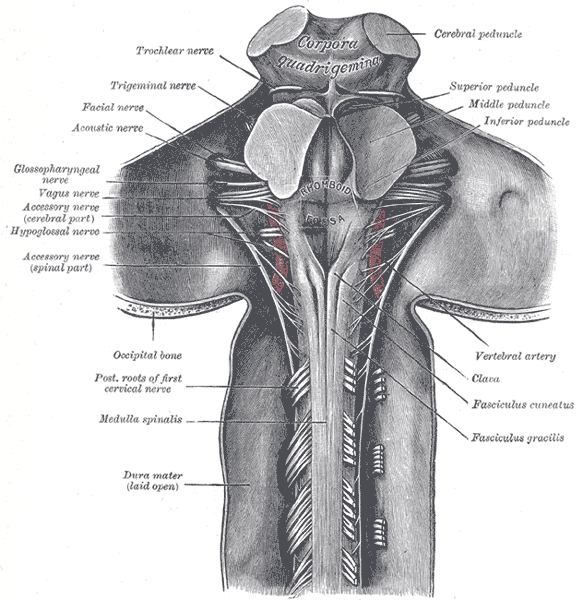
Glossopharyngeal Nerve (IX): This nerve has three functions from three different nuclei, the main motor nucleus, parasympathetic nucleus, and sensory nucleus. The motor nucleus is located in the nucleus ambiguous, found within the reticular formation of the medulla, just below the pontomedullary junction. The nerve runs through the subarachnoid space, exiting the skull through the jugular foramen. The motor component of the glossopharyngeal nerve only supplies the stylopharyngeus muscle, which contributes to the gag reflex and helps elevate the pharynx when talking or swallowing. The parasympathetic preganglionic fibers originate from the inferior salivatory nucleus, located in the pons. These fibers synapse in the otic ganglion and proceed to innervate the parotid glands to stimulate salivation. The sensory fibers of the glossopharyngeal nerve innervate the chemoreceptors and baroreceptors of the carotid body and send their information to the caudal nucleus solitaries. Cranial nerve IX sensory fibers also innervate the posterior one-third of the tongue, carrying taste, touch, and pain information to the rostral nucleus solitaires.
Vagus Nerve (X): The tenth cranial nerve contains four nuclei in the medulla. The dorsal motor nucleus of the vagus nerve, near the floor of the fourth ventricle, is the largest nuclei of the vagus nerve, and it runs from the rostral to the caudal medulla. The dorsal nucleus receives afferent fibers from the hypothalamus and has efferent fibers sending parasympathetic signals to the heart, stomach, bronchi, esophagus, pancreas, and proximal intestines. The motor nucleus is within the nucleus ambiguous, which is within the reticular formation of the medulla. The vagal fibers from the nucleus ambiguous innervate the muscles of the palate, pharynx, upper esophagus, and larynx and stylopharyngeus. The motor innervation controls speech and swallowing with the help of the glossopharyngeal. The general sensory fibers of the vagus that receives information from the pharynx, larynx meninges of the posterior fossa, and the region near the external auditory canal reach the caudal nucleus solitaries. The general visceral sensation from the chemoreceptors and baroreceptors of the aortic arch and digestive tract is carried back to the nucleus solitaries as well. The gag reflex, cough reflex, and carotid sinus reflex occur via the combination of sensory and motor fibers of the vagus nerve from the nucleus solitaries. The taste sensation of the epiglottitis and posterior pharynx gets carried to the rostral nucleus solitaries.[12]
Accessory Nerve Nucleus (XI): The accessory nerve is the combination of a cranial nerve arising from the medulla and cervical roots up to C5. The cranial nerve nucleus starts from the nucleus ambiguous. Nerve rootlets until C5 leave the lateral aspect of the spinal cord between the dorsal and ventral nerve roots and ascend through the foramen magnum to exit back through the jugular foramen and supply the sternocleidomastoid and trapezius muscles.
Hypoglossal Nerve (XII): The hypoglossal nucleus is situated midline near the vagus nerve nuclei and caudal to the bottom of the fourth ventricle. It produces a bulge in the floor of the fourth ventricle, named the hypoglossal eminence/trigone. The hypoglossal nerve supplies the intrinsic muscles of the tongue and the styloglossus. A lesion in the hypoglossal nerve nuclei causes ipsilateral tongue weakness, which manifests as the tongue deviating towards the weak side.
Surgical Considerations
The medulla has always been considered challenging to surgically treat, due to its essential cardiac, respiratory, and autonomic functions. Medulloblastoma, glioma, ependymoma, and hemangioblastoma are common intracranial germ cell tumors that originate from extragonadal seminal cells and usually are located in midline structures but rarely in the medulla and the fourth ventricle. Symptoms typically include cranial nerve palsies such as dysphagia, disturbance of breathing, cerebellar function, motor, sensory disturbances, and hiccups. From 1991 to 2013, there were only 17 cases of pure primary medulla oblongata germinoma. Using a CT and MRI, clinicians diagnosed the medullary mass. Patients received tumor debulking as their initial management. After the initial surgery, patients had the option to be treated with radiotherapy and chemotherapy, which both led to a good prognosis.[13]
Cavernous malformations have been found to develop in the brainstem, and rare cases can affect the medulla. Cavernous malformations involving the medulla oblongata have been reported to be more harmful if bleeding occurs due to the major functions located in the medulla. Small changes or injury to the medulla can lead to paraplegia, cardiovascular and respiratory dysfunction, or vagus nerve injury. There is still a debate in the community on which patients will benefit from surgical treatment. Out of 69 case reports of cavernous malformation located in the medulla, three distinct microsurgical approaches applied to the resection involved the medulla oblongata. The far lateral craniotomy technique was used for lesions in the lateral or anterolateral portion, while the rectosigmoid craniotomy was the option chosen for lesions involving the pontomedullary. Lesions in the posterior medulla or the fourth ventricle had a suboccipital craniotomy performed. Postoperatively, patients require observation for at least 48 hours after surgery because of the high risk of respiratory dysfunction. About 30% of the patients had immediate post-operative dysphagia or coughing. Ming-Guo Xie et al. in 2018 found a favorable functional long-term outcome with younger patients, who have experienced fewer hemorrhages, and perilesional edema. They believed perilesional edema findings around the cavernous malformation on the MRI should be considered a protective factor because edema around the malformation can signal a more favorable prognosis in response to surgical intervention. These patients would benefit more from surgical resection when compared to patients without perilesional edema because the resection could relieve the pressure and improve the blood supply to the brainstem for a functionally improved long term outcome.[14]
Clinical Significance
Lateral Medullary Syndrome (Wallenberg syndrome): This is the most common stroke in the medulla. It is due to vertebral artery thrombosis or dissection. A lesion in the lateral medulla produces ipsilateral ataxia (inferior cerebellar hemisphere and spinocerebellar fibers), vertigo, horner syndrome (sympathetic fibers), headache, horizontal or vertical nystagmus (inferior vestibular nucleus and pathway), dysphagia, dysphonia (fibers of the cranial nerve IX and X), and pain with impaired facial sensation on the face (descending spinal trigeminal complex fibers). On the contralateral side, there is impaired pain and temperature sensation of the arms and legs due to the involvement of the spinothalamic tract. This syndrome does not include weakness because the corticospinal fibers are not affected. It is associated with occlusion, most likely due to atherothrombosis of the posterior inferior cerebellar artery or its branches supplying the lateral medulla.[15][16][15]
Medial Medullary Syndrome (Dejerine syndrome): This results from occlusion of the distal vertebral artery or the anterior spinal artery. Lesions cause a lack of blood flow to the pyramidal decussation resulting in a contralateral arm and leg paralysis. The contralateral face has also been found to be affected in 50% of cases. Ipsilateral tongue paralysis can occur due to the hypoglossal nerve, and nystagmus can also present from the damage of the medial longitudinal fasciculus.
Bilateral medial medullary syndrome: This is an uncommon combination of the two major syndromes, affecting bilateral medial medullary. The associated symptoms are flaccid quadriplegia sparing the face, hypoglossal nerve palsy, and respiratory failure.
Hemi medullary syndrome (Reinhold): This is a very uncommon disease, with around ten patients reported in the medical literature. It has lateral and medial medullary syndrome symptoms. This lesion results in contralateral hemiparesis, contralateral hemisensory loss, ipsilateral ataxia, ipsilateral Horner’s syndrome, ipsilateral facial sensory loss, dysarthria, nausea, and vomiting.[17]
Babinski-Nageotte and Cestan–Chenais: These are very rare intermediolateral syndromes that can be considered “halfway state” between Wallenberg lateral and Reinhold hemi-medullary syndrome. Babinski-Nageotte has all the symptoms of lateral medulla syndrome with hemiparesis because the lesion has spread to the pyramidal tracts. Babinski-Nageotte syndrome is the result of simultaneous ischemia of the branches from the PICA and the anterior spinal artery, which is why it is so uncommon. Cestan-Chenias syndrome occurs with infarction of the lateral and anterolateral arterial groups with sparing of the posterior arterial group area. This syndrome has all the symptoms of Babinski-Nageotte syndrome, but there is no cerebellar ataxia because it spares Flechsig’s afferent posterior spinocerebellar tract.