Introduction
The mediastinal compartment contains multiple critical organs and vessels and serves as the central hub for lymphatic drainage. The mediastinum is classically subdivided into three functional divisions: anterior (pre-vascular), middle (visceral), and posterior (paravertebral) mediastinum. These subdivisions are used to describe the locations of lesions, thereby helping to facilitate differential diagnoses and communication between providers. Lymph nodes (LNs) are present in all three functional compartments of the mediastinum, though most lymphatic tissue is found in the anterior and middle compartments, and the etiology of lymphatic pathology varies by subdivision. Dividing the mediastinum helps to narrow down the lengthy differential diagnoses, which can present in the thorax (including, but not limited to: infections like tuberculosis, the nodal spread of lung cancer, sarcoidosis, lymphoma, silicosis, and asbestosis). In contrast to the functional subdivisions, intrathoracic LN locations have been traditionally mapped into 14 stations according to their relationship to landmarks encountered during mediastinoscopy and thoracotomy for lung cancer. Stations 1–9 correspond to mediastinal nodal groups, while stations 10–14 represent hilar and other more peripheral extra mediastinal nodal groups.
The most current map of intrathoracic lymph nodes is the International Association for the Study of Lung Cancer (IASLC) map. The IASLC Atlas supersedes all previous schema and reconciles discrepancies among older popular systems such as the Naruke lymph node classification and the Mountain-Dresler modified version of the American Thoracic Society lymph node map.[1][2][3][4]
Structure and Function
The network of lymphatic vessels and LNs extends throughout the entire body to support the proper function of its immune system along with the absorption of dietary fats and fluid homeostasis. Unlike arteries and veins, lymphatics are blind-ending vessels that transport interstitial fluid, immune cells, and macromolecules, collectively called lymph, through LNs and back to the chest for return into the blood supply via the subclavian veins. Lymph originates when blood plasma leaks out of capillaries and into the interstitial space. Along with the normal macromolecules and lymphocytes within the plasma, dead cells and material that may be harmful to the body such as bacteria, viruses, and tumor cells also leak out, and in turn, enter the lymph. The fluid and cells are transported back to the chest by the lymphatic vessels. Along the way, nodes, like those found in the mediastinum, sample the lymph, filtering out potential threats and unwanted cellular debris before it re-enters the blood supply. Macrophages within the lymph nodes phagocytize these unwanted substances and then break them down for recycling by the body. T-lymphocytes sample antigens from pathogens and interact with B cells to initiate clonal expansion for antibody production. With immune cell activation, LNs grown as cells within the divide. Rapid growth can cause pain, while slow mitotic activity (often seen with cancer) results in painless lymphadenopathy.[5][6]
Embryology
Lymphatic system development begins at the end of the fifth week of embryogenesis, approximately 2 weeks after the development of the cardiovascular system. While still somewhat controversial, the most prevalent theory is that the lymphatic system begins as channels forming from the diverticula off the venous endothelium. During weeks 6 through 9, focal dilations of the channels result in the creation of six primary lymph sacs. Lymph vessels then grow out from the sacs along the major veins. At about week 12 of fetal life, much of the lymph sacs are transformed into groups of lymph nodes while additional LNs form along the lymph vessels, while a small portion of the sacs goes on to contribute to the formation of the cisterna chyli and thoracic duct.[7]
Blood Supply and Lymphatics
The organization of mediastinal lymph nodes is based upon their relation to surgical landmarks: great vessels, trachea/bronchi, and pleura. On CT, the preferred radiologic modality for visualizing lymph nodes, the normal mediastinal nodes are reniform soft tissue structures with a fatty hilum. Normal lymph nodes in the mediastinum typically measure less than 10 mm by short axis. Healthy lymph nodes can be larger, due to reactive hyperplasia from acute infection or chronic lung diseases such as emphysema or pulmonary fibrosis; however, enlarged lymph nodes are most worrisome for a pathologic process such as lymphoma, malignant metastases, or sarcoidosis.
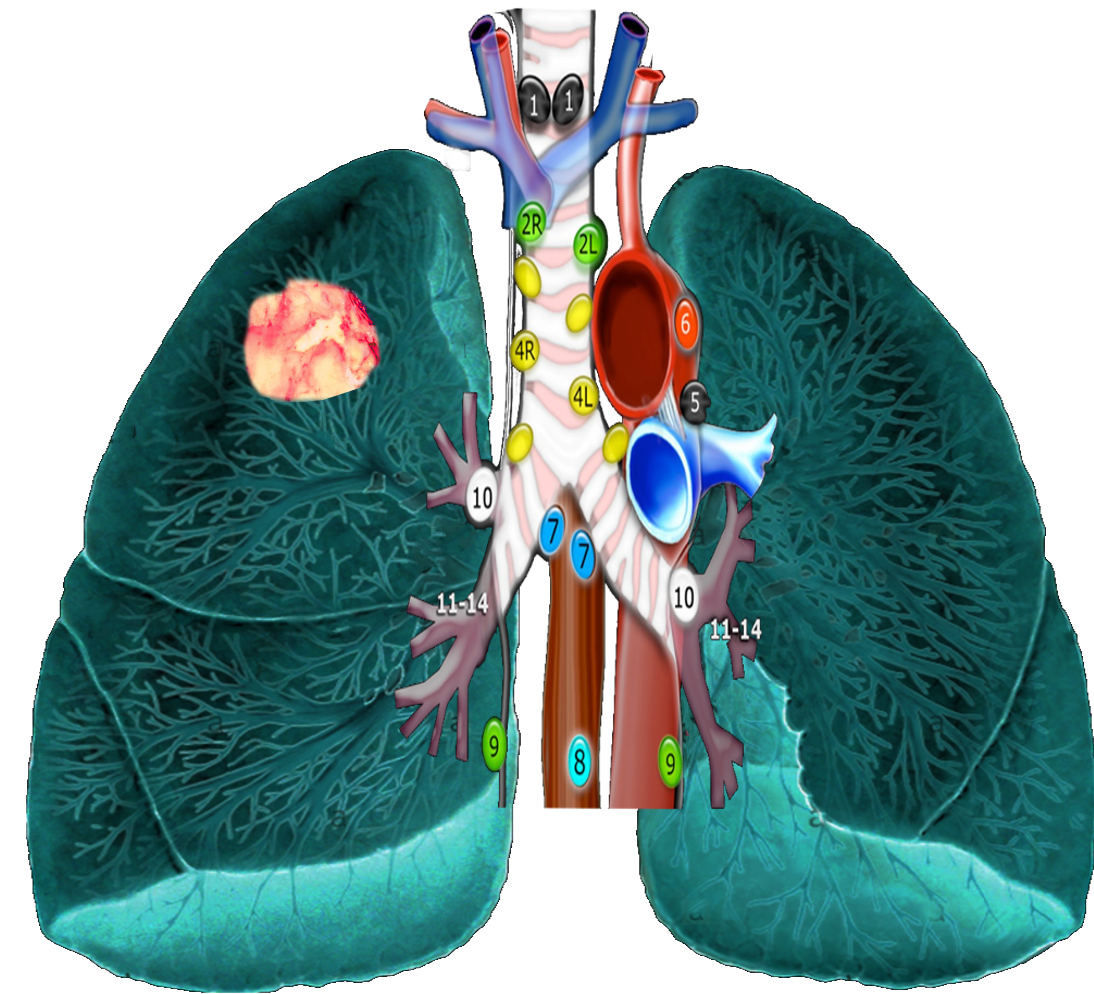
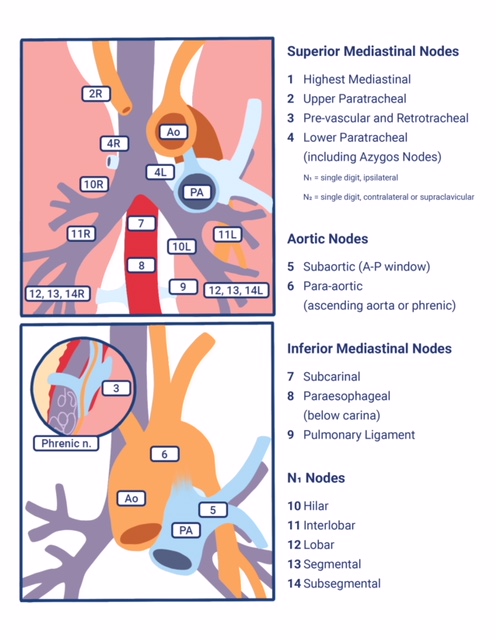
As mentioned, based on lung cancer staging guidelines, the intrathoracic lymph nodes are divided into 14 stations, which are grouped into 7 zones. Stations 1–9 are located in the mediastinal pleural reflection, while stations 10–14 are distal to the mediastinal pleural reflection and within the visceral pleura. Stations 1, 2, 4, and 10-14 additionally have R and L designators for right and left, while station 3 has A and P designators for anterior and posterior. The intrathoracic are frequently considered in conjunction with the mediastinal lymph nodes, but extra mediastinal lymph nodes (stations 10-14) will be discussed briefly in this article for completeness.
Lymph Node Zones and Stations
Supraclavicular Zone
Station 1 (Supraclavicular): Station 1 comprises the most cranial station of mediastinal nodes. It includes LNs in the sternal notch, supraclavicular, and lower cervical regions (thus overlapping with some cervical LN maps used in head and neck cancers). The cricoid cartilage serves as the upper border of station 1. Station 1 extends inferiorly to the upper margin of the manubrium and tops of the clavicles. The midline of the trachea is used to designate which lymph nodes are 1R and 1L.
Upper Zone (Superior Mediastinal LNs)
Station 2 (Upper Paratracheal): Station 2 LNs all about the trachea and, in contrast to station 1, the left lateral wall of the trachea instead of the midline, is used as the boundary to differentiate between 2R versus 2L. The upper border of station 2 is the apex of the ipsilateral lungs and pleural spaces, and in the midline, the upper border of the manubrium. The lower border of station 2 on the right (2R) is where the inferior margin of the left brachiocephalic vein crosses the trachea, while the lower border of station 2 on the left (2L) is the superior border of the aortic arch.
Station 3 (Prevascular and Retrotracheal): The prevascular lymph nodes (3A) are all located behind the sternum and anterior to the superior vena cava and left carotid artery. The superior border is the apex of the chest (like station 2) but extends further caudal to the level of the carina. Retrotracheal LNs (3P), as their name implies, are those located in the area posterior to the trachea, likewise extending from the apex of the chest to the carina.
Station 4 (Lower Paratracheal): Lower paratracheal nodes are along the distal trachea, bordered superiorly by station 2, and extending to the level of the carina. They lie posterior to the aortic vasculature, and like station 2, the left lateral wall of the trachea instead of the midline is used as the boundary to differentiate between 4R and 4L.
Aortopulmonary Zone
Station 5 (Subarotic): These lymph nodes are also known commonly as aortopulmonary (AP) window LNs and are located lateral to the ligamentum arteriosum, the remnant of the ductus arteriosus. The lower margin of the aortic arch serves as the upper border of station 5, while the superior margin of the left pulmonary artery demarcates the lower extension.
Station 6 (Paraaortic): The para-aortic LNs lie on the anterior and lateral aspect of the ascending aorta and aortic arch, anterior and/or above the subaortic (AP window) LNs. The phrenic nerve may be used as a landmark for identifying lymph nodes that are classified as paraaortic.
Subcarinal Zone
Station 7 (Subcarinal): Subcarinal nodes lie directly below the carina and between the mainstem bronchi. To differentiate them from the paraesophageal LNs that are found more caudal, the distal aspect of the bronchus intermedius and origin of the left lower lobe bronchus is used to demarcate the right and left inferior extensions of station 7. In most patients, this results in an inferior margin that is canted from the horizontal, given that the termination of the bronchus intermedius is usually lower than the origin of the left lower lobe bronchus).
Lower Zone (Inferior Mediastinal LNs)
Station 8 (Paraesophageal): Paraesophageal nodes are those mediastinal lymph nodes found inferior to the subcarinal lymph nodes, along with the anterior or lateral aspects of the esophagus, down to the esophageal hiatus of the diaphragm.
Station 9 (Pulmonary ligament): Pulmonary ligament nodes are associated with the pulmonary ligaments. These “ligaments” are not ligaments but represent the mediastinal parietal pleural reflections that occur below the right and left pulmonary roots (9R and 9L).
Hilar Zone + Interlobar and Peripheral Zone (Extra-Mediastinal LNs)
These lymph nodes are all outside the pleural reflection of the mediastinum but within the pulmonary visceral pleura.
Station 10 (Hilar): These LNs are found along the right and left mainstem bronchi, before they bifurcate, and are designated 10R and 10L, respectively.
Station 11 (Interlobar): Station 11 is made up of LNs located between the lobar bronchi, just beyond the bifurcation of each mainstem bronchi.
Stations 12-14 (Peripheral): These are also known as lobar, segmental, and subsegmental lymph nodes, depending on whether they are located along the lobar, segmental, or subsegmental bronchi. These LNs are infrequently seen and difficult to accurately categorize on imaging; hence many use the broad term of peripheral LNs for stations 12-14.
It should be noted that while the above classification system is the most widely utilized mapping scheme and often employed when describing mediastinal lymph nodes outside the setting of lung cancer, other disease-specific maps are appropriate in certain instances such as esophageal cancer staging.[1][8][9][10]
In addition to mediastinal lymph nodes, the thoracic duct is an important component of the intrathoracic lymphatic system. It is the largest single lymphatic vessel in the chest, beginning at the superior aspect of the cisterna chyli, at the level of the L2 vertebra. From there, it courses cranially between the posterior margin of the aorta and anterior margin of the spine until approximately the region of the T5 vertebra, where it drains into the venous system near the junction of the left subclavian and internal jugular veins. Approximately 75% of the body’s lymph fluid drains via the thoracic duct into the venous system, accounting for lymphoid drainage from the entire body, except for the right arm and right side of the head (the nodes of which drain into the junction of the right subclavian and internal jugular veins).[11][12][13]
Physiologic Variants
The budding of lymphatics from veins can be very inconsistent between individuals, and thus nodal chains demonstrate widespread variability. Notably, the most commonly identified course of the thoracic duct is present in only 40% to 60% of patients. Common presentations include thoracic ducts and corresponding cisterna chyli that present on the anatomic left, which drain into the left venous angle; thoracic ducts and their corresponding cisterna chyli that present on the anatomic right, which drain into the right venous angle; plexiform presentation; proximal and distal partial duplications of the thoracic duct; and nonexistent cisterna chyli.[13]
Surgical Considerations
Mediastinal lymph node sampling is critical to the initial diagnosis and staging of lung and esophageal cancers and lymphoma. Additionally, it is essential for detecting primary or recurrent nodal metastases in extrathoracic malignancies. This can be done by a variety of techniques employed by a variety of specialists with the technique usually guided by the location of nodal disease. Cardiothoracic surgeons may employ mediastinoscopy for the sampling of stations 1, 2R/L, 3, 4R/L, and 7. An extended mediastinoscopy or video-assisted thoracotomy (VATS) can extend their range to include biopsy of stations 5 and 6. Gastroenterologists may use esophageal ultrasound (EUS), which allows for fine-needle aspiration (FNA) of mediastinal lymph node stations 2L, 4L, 7, 8, and 9. Pulmonologists can use transbronchial needle aspiration (TBNA), as well as endobronchial ultrasound (EBUS) FNA, which allows for tissue sampling of the bilateral station 10-12 LNs (hilar and peripheral stations) in addition to 3P, station 7, and bilateral station 2 and 4 LNs. Similarly, these techniques can be used for the diagnosis of other disorders that may involve the mediastinal lymph nodes such as sarcoidosis, Castleman disease, or tuberculosis.
In addition to cancer staging, mediastinal lymphatic disorders can have other surgical implications. Chylous pleural effusions can occur when the intrathoracic lymphatic tissue is damaged, either as a result of infection/inflammation or trauma/surgery and lymph leaks into the pleural spaces. Less commonly, a chylopericardium can result if the insulting event results in a fistulous connection between the lymphatics and pericardium. If embolization of the lymphatic system is indicated after a chylothorax develops, magnetic resonance ductography has the best visualization to guide surgical intervention, but CT scans, lymphoscintigraphy, and lymphangiography can be used alternatively.[13][14][15][16][17]
Clinical Significance
In the setting of primary intrathoracic tumors, it is critical to accurately identify the station from which lymph nodes are excised/sampled, since information regarding which LNs the tumor has spread to is essential to the surgical staging of a patient’s regional disease. In the case of lung cancer, tumor involvement of ipsilateral station 10-14 lymph nodes, all of which are extrapleural, is considered N1 disease, while mediastinal LNs from stations 2-9 that are ipsilateral to the malignancy (to include subcarinal LNs for either side of disease) are considered N2 disease. Contralateral mediastinal or hilar lymph nodes, as well as station 1 LNs that have tumor cells present, are considered N3 disease. In contrast, with esophageal cancer, the N stage of the disease is determined by the number of regional LNs involved by tumor with N1 being 1-2 positive (+) LNs, N2 employed for 3-6 +LNs, and N3 designating cases with >=7 +LNs. Additionally, not all nodal stations used by the IASLC for lung cancer are considered regional LNs for esophageal cancer staging, and some stations are named differently, such as the designation of the upper, middle, and lower paraesophageal LN stations.
In addition to metastases, malignant mediastinal lymph nodes can be due to lymphomas, a group of cancers that arise from white blood cells. This group of diseases is the most frequent cause of the nodal pathology of the anterior compartment but is also a common cause of adenopathy in the middle compartment.
Mediastinal adenopathy may be due to non-neoplastic entities. Primary acute or chronic infectious and inflammatory processes occur in all compartments of the mediastinum and may result in lymph node enlargement. Likewise, pulmonary and systemic infections such as pneumonia and the human immunodeficiency virus can result in pathologically enlarged mediastinal lymph nodes. If mediastinal lymph nodes are calcified, it helps narrow the differential diagnosis to entities such as granulomatous diseases (histoplasmosis, tuberculosis), silicosis, sarcoidosis, and treated lymphoma. When lymphatic vessels of the mediastinum are damaged, the resulting chylothorax can interfere with normal immunologic, nutritional, and respiratory function.
Enlarged mediastinal lymph nodes may be found incidentally, and guidelines for surveillance and evaluation can be found in the Journal of the American College of Radiology. For example, nodes may be particularly worrisome if they are large, asymmetric, or demonstrate FDG avidity on PET/CT scans. Nodes that are larger than 15 mm may warrant special attention, especially if there is no known explanation, such as a history of sarcoidosis. The American College of Radiology guidelines also recognize the value of categorizing lymph nodes by the aforementioned divisions, identifying abnormal LN contours, and whether the masses are solid or cystic.[3][8][12][13][18]