Introduction
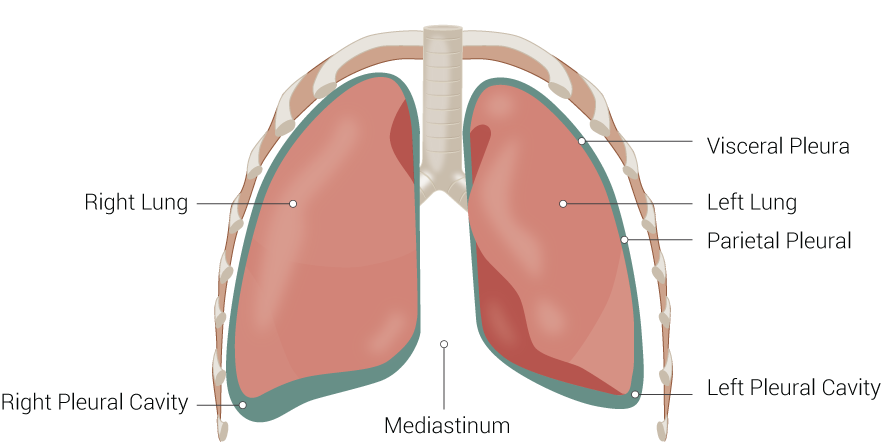
Pulmonary compliance, a measure of the expansion of the lung, is critical to the proper function of the respiratory system. Lung compliance can be calculated by dividing volume by pressure. Factors affecting lung compliance include elasticity from the elastin in connective tissue and surface tension, which is decreased by surfactant production. Lung compliance participates in the lung-chest wall system by opposing the outward pull of chest wall compliance. See Image. Lung Anatomy. The net compliance (lung-chest wall system) allows the lungs to achieve appropriate functional residual capacity, the volume remaining after passive expiration.
Cellular Level
Anatomically, the respiratory tract divides into 2 zones: the conducting and respiratory zones. These 2 lung zones contain different cells to support the specialized functions of the respective regions. The conduction zone conditions and facilitates the movement of air entering the lungs. This zone mostly contains a respiratory epithelium that is ciliated or pseudostratified columnar. This dominating cell type helps moisturize and filter the air. The 5 types of specialized cells in the conducting zone include ciliated cells, goblet cells, basal cells, brush cells, and neuroendocrine cells.[1][2][3]
- Ciliated cells are the most plentiful cell types and function to remove debris.[4]
- Goblet cells trap and destroy micro-particles by mucin granules. This cell type decreases in number down the respiratory tree and eventually becomes replaced with club cells.[3]
- Basal cells connect ciliated and goblet cells to the basement membrane.[5]
- Brush cells (also known as type 3 pneumocytes): Columnar or pear-shaped cells with microvilli and unmyelinated nerve ending throughout a small portion of the mucosal epithelium. The function is uncertain. However, some literature indicates a possible chemoreceptor function that monitors air quality.[6]
- Neuroendocrine cells (Kulchitsky cells) are located in the bronchial submucosa and secrete catecholamines and polypeptide hormones (serotonin, calcitonin, and gastrin-releasing factor).[3]
The respiratory zone includes the alveolar epithelium, which has a lining with 3 types of pneumocytes.
- Type 1 Pneumocytes: squamous cells line most alveolar surfaces to promote optimal gas diffusion.[7]
- Type 2 Pneumocytes: secrete pulmonary surfactant, which ultimately decreases alveolar surface tension to prevent alveolar collapse, decrease elastance, and increase compliance. These cells are precursors to type-1 cells and other type-2 cells. This ability to proliferate makes type 2 pneumocytes helpful during lung damage.[7]
- Club cells (previously known as Clara cells): nonciliated, low-columnar/cuboidal cells with secretory granules. Club cells secrete part of the surfactant, degrade toxins, and act as regional progenitor cells in conditions of lung damage.[8]
Development
The endoderm gives rise to the epithelial layer of the lungs, while the mesoderm gives rise to the connective tissue. Lung development subdivides into 5 distinct stages: embryonic (weeks 4 to 7), pseudo glandular (weeks 5 to 7), canalicular (weeks 16 to 25), saccular (week 26 to birth), and alveolar (weeks 36 to 8 years).[9][3][10]
Embryonic Stage: Development of the lungs begins in the fourth week: lung buds stem from the distal end of the respiratory diverticulum. The respiratory diverticulum forms from the ventral foregut. The 2 lung buds further divide into 2 bronchial buds. In week 5, bronchial buds branch into the main (primary) bronchi. By the end of the embryonic stage, the lobar (secondary) and segmental (tertiary) bronchi also branched off. Simultaneously, 2 pulmonary arteries grow from the sixth aortic arch, forming a vascular plexus.[3][10][11][10]
Pseudoglandular Stage: Between weeks 5 and 16, terminal bronchioles and surrounding capillary network forms. The terminal bronchi mark the end of the development of the conducting zone and the beginning of the respiratory zone, which includes the respiratory bronchioles and alveolar sacs. Moreover, mesenchymal cells differentiate into cartilage and smooth muscle cells. Smooth muscle cells, fibroblasts, chondrocytes, and endothelial cells are known to produce elastin.[3][10][12][10]
Canalicular Stage: Respiratory bronchioles, alveolar ducts, and a stronger capillary network develop between weeks 16 and 25. Alveolar ducts support the growth of lung parenchyma, which acts as the site for gas exchange. Additionally, airways develop during this stage, and pneumocytes (types 1 and 2) develop at 20 weeks.[3][10]
Saccular Stage: Alveolar ducts branch into terminal sacs between week 26 and birth. The increase of fetal cortisol during this stage also leads to the maturation of the lungs (tissue remodeling and cell differentiation) and the synthesis and secretion of surfactant. More specifically, the extracellular matrix (ECM) maturation is a critical event that leads to proper lung compliance. During ECM maturation, stability takes place through elastin and collagen cross-linking.[3][10][13]
Alveolar Stage: Alveoli continue to develop and proliferate after birth to about 8 years of age.[3] The lung divides into 2 lobes on the left and 3 on the right, visible on gross anatomy. See Figure. The Lungs.
Function
The lung mainly functions in 2 ways: conduction and respiration. As previously described, these functions can be examined in particular zones of the lung: conduction zone and respiratory zone. Conduction involves the warming and filtration of air, while respiration includes the exchange of oxygen and carbon dioxide. Respiration highly depends on lung and chest wall compliance, airway resistance, and ventilation rate. Together, these components allow an A-a gradient to guide air appropriately into the lungs on inhalation and exhalation.
Compliance of the respiratory system describes the expandability of the lungs and chest wall (see Graph. Lung Compliance). There are 2 types of compliance: dynamic and static. Dynamic compliance describes the compliance measured during breathing, which involves a combination of lung compliance and airway resistance. Static compliance describes pulmonary compliance with no airflow, like an inspiratory pause. Pressure-volume curves are common schemes to express the relationship between dynamic and static compliance where the slope is compliance.[14]
C = V/P
- C: Compliance (ml/mmHg)
- V: Volume (mL)
- P: Pressure (mm Hg)
Lung compliance is the lung volume change for a given change in transpulmonary or transmural pressure. The transmural pressure (PTM) is the difference between intrapleural pressure (PA) and alveolar pressure (Pa) [PTM= PA – Pa]. If the intrapleural pressure is more negative, the lungs increase in volume to expand. However, if the intrapleural pressure is positive, the lungs collapse, which decreases lung volume. During expiration, the lung volume is higher for a given intrapleural pressure; therefore, compliance is higher in expiration than inspiration. Lung compliance is inversely related to elastance, elastic resistance, or recoil. So, patients with low lung compliance have relatively stiff lungs and, therefore, higher elastance.[15]
Two important factors of lung compliance are elastic fibers and surface tension. More elastic fibers in the tissue increase expandability and, therefore, compliance. Surface tension within the alveoli is decreased by surfactant production to prevent collapse. Compliance is more easily achieved by decreasing surface tension.
The lung and chest wall, together, form a combined compliance system. Independently, each lung and chest wall measures higher compliance than the combined lung-chest wall system. In addition to lung compliance, the combined system factors in the opposing force of the chest wall muscles and diaphragm. These muscles provide the necessary pressure difference for air movement. The combined lung-chest wall system is at equilibrium (no inclination toward collapse or expansion) when lung volume is at functional residual capacity (FRC), the remaining lung volume after tidal volume has expired. The 2 opposing forces of the chest and lungs set the negative intrapleural pressure.[16]
Related Testing
Mechanical Ventilation: Pulmonary compliance is directly measurable in a mechanically ventilated patient. For accuracy, airway resistance is removed as a contributing factor by taking static measurements instead of dynamic ones. The examiner records end-expiratory and end-inspiratory alveolar pressure to determine static measurements. The tidal volume, the change in lung volume, is divided by the change in pressure. After plotting these measurements, the slope reveals lung compliance. Measurement of compliance is useful when monitoring critically ill patients at the bedside.[17]
Pulmonary Function Tests (PFTs) are a group of noninvasive tests that measure lung function. PFTs are not tests of diagnosis, but they help support a diagnosis by supplementing detailed history, physical exam findings, and laboratory results. An understanding of lung compliance is necessary to interpret and use PFT results.[14][18]
Pathophysiology
Restrictive lung diseases - fibrosis and interstitial lung disease: In interstitial lung diseases, the lung and/or chest wall compliance has decreased. Therefore, there is an increased tendency for the lungs to collapse. Because of the restriction on lung expansion, there is also a reduced lung volume (low FVC and TLC). Since the tendency to collapse is high at the original FRC, the lung-chest wall system seeks a lower FRC to balance the opposing forces. PFTs usually reveal a high FEV1/FVC ratio. FEV1 is the volume of air that is forcefully expired in the first second.
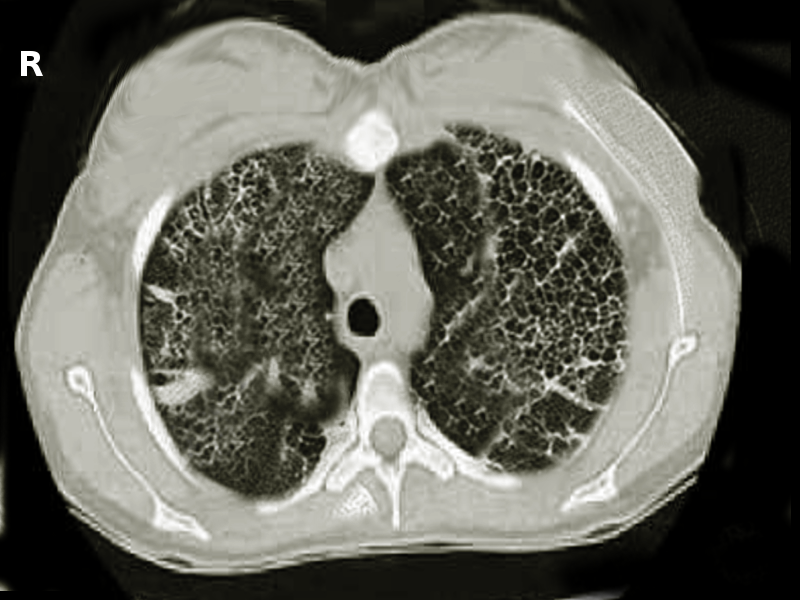
Values of 80% (0.8) of the average are considered normal. Restrictive lung diseases include fibrosis and interstitial lung diseases (pneumoconiosis, sarcoidosis, etc). See Image. Lung Fibrosis. Pulmonary mechanical issues can also lead to a restriction in lung expansion.
Restrictive lung disease can result from mechanical issues with peripheral hypoventilation, including poor muscular effort or structural dysfunction. Conditions like muscular dystrophy, polio, myasthenia gravis, and Guillain-barre syndrome can cause poor muscular effort. Muscular dystrophy reduces the ability of the lung to distend for unknown reasons, which reduces lung compliance. However, although there is an impact, respiratory muscle weakness or tension is not directly related to pulmonary compliance. Scoliosis or morbid obesity can also cause structural limitations.[23]
Obstructive lung disease: When airflow obstructs, the air path becomes halted earlier than expected at high lung volumes. This condition leads to trapped air in the lungs. Compliance can be normal or increased. PFTs usually reveal a low FEV1/FVC ratio as FEV1 is reduced significantly more than FVC. Again, since the tendency to collapse is high at the original FRC, the lung-chest wall system seeks a lower FRC to balance the opposing forces. Obstructive lung diseases include asthma, chronic obstructive pulmonary disease (COPD), chronic bronchitis, and emphysema.[24]
- Asthmatic patients present with cough, wheezing, dyspnea, tachypnea, hypoxemia, and mucus plugging. Asthma is characterized by hypersensitive bronchi (a type 1 hypersensitivity reaction). Diagnosis can be made by spirometry and followed by a methacholine challenge.
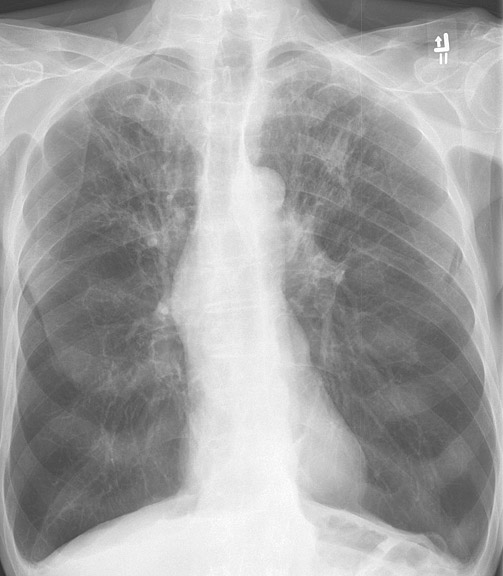
- COPD is categorized into 2 conditions: chronic bronchitis and emphysema (see Image. X-Ray, Chest, Chronic Obstructive Pulmonary Disease, Anterior).
- Patients with chronic bronchitis are also known as blue bloaters. They present with wheezing, dyspnea, crackles, cyanosis, CO2 retention, and/or secondary polycythemia. There is usually a history of chronic productive cough because of hyperplasia and hypertrophy of glands secreting mucus in the bronchi.[16]
- Patients with emphysema are also known as pink puffers. A classic finding in these patients is a barrel-shaped chest, which is apparent on a chest X-ray. DCLO decreases due to the destruction of alveolar walls. The most common risk factor is smoking, which leads to a loss of elastic fibers and increased lung compliance. A common technique to relieve symptoms is breathing with pursed lips to increase pressure in the airways and prevent collapse.[16]
There are cases of mixed obstructive and restrictive lung diseases where there is a premature formation of segments that restrict airflow. Also, there is low lung compliance, which leads to a decrease in FVC.
Clinical Significance
Pneumothorax: This condition characteristically demonstrates the accumulation of air in the intrapleural space. This leads to the equalization of intrapleural pressure with atmospheric pressure. As a result, the chest wall protrudes outward, and the lungs collapse, as this is the natural tendency without transmural pressure. The patient commonly presents with dyspnea, chest pain, uneven expansion of the chest, and diminished breath sound on the affected side. Without proper transmural pressure, lung-chest wall compliance is inoperable.[25]
Obesity results in low lung compliance reduced functional residual capacity, and expiratory reserve volume.[26]
Scoliosis decreases the chest wall and lung compliance, increasing respiratory workload.
Aging is accompanied by a decrease in muscular strength and elastic recoil. Therefore, lung compliance increases, and chest wall compliance decreases as age increases.[27]