Continuing Education Activity
Lichen planus is an inflammatory condition of unknown etiology affecting the skin and mucous membranes. This condition typically presents as itchy, violaceous papules and plaques commonly located on the wrists, lower back, and ankles. A distinctive lattice of white lines, known as Wickham striae, often overlays the lesions and is most easily visible on the buccal mucosa, where erosions may also occur. Drug-induced lichen planus, or lichenoid drug eruption, can resemble idiopathic lichen planus, often displaying a photo-distributed pattern, making differentiation between the 2 forms challenging.
The natural course of lichen planus varies widely. Most patients with cutaneous lesions experience spontaneous resolution within 1 to 2 years of onset. However, recurrences are common, and residual skin hyperpigmentation often persists. In contrast, oral lichen planus tends to be a chronic condition that may or may not remit over time. Drug-induced lichen planus typically improves gradually after discontinuation of the offending medication.
This activity for healthcare professionals describes the epidemiology, genetics, diagnostic approaches, treatment options, and management strategies for cutaneous and oral forms of the disease. The activity extensively reviews the clinical presentation, including hallmark signs and the differentiation between idiopathic and drug-induced lichen planus. This activity also highlights the role of an interprofessional team in improving patient care for individuals with lichen planus and optimizing long-term management to prevent recurrences.
Objectives:
Differentiate between idiopathic and drug-induced lichen planus based on clinical presentation and patient history.
Screen patients for potential triggers of lichen planus, including medications, allergens, and other environmental factors, to guide appropriate management.
Select evidence-based treatment strategies to reduce recurrence and manage cases of lichen planus.
Implement effective collaboration and communication among interprofessional team members to improve outcomes and treatment efficacy for affected patients.
Introduction
Lichen planus is an inflammatory disorder affecting the skin and mucous membranes with no known cause. The condition appears as pruritic, violaceous papules and plaques most commonly found on the wrists, lower back, and ankles. The lesions are often overlaid with a lattice-like network of white lines called Wickham striae, which are most easily observed on the buccal mucosa, where erosions may also be present. Drug-induced lichen planus, or lichenoid drug eruption, is frequently photo-distributed but may be indistinguishable from idiopathic lichen planus.[1][2]
The natural course of lichen planus varies significantly. Most patients with cutaneous lesions spontaneously clear within 1 to 2 years after initial presentation.[3] However, recurrences are common, often resulting in residual skin hyperpigmentation. In contrast, oral lichen planus is a chronic disease that may or may not remit.[4] Treatment-induced remission is typically followed by relapse. Thus, asymptomatic oral lichen planus should not be treated due to the high burden of treatment-related adverse effects. Drug-induced lichen planus gradually resolves once the causative medication is discontinued.[5]
Etiology
Lichen planus is an idiopathic disease whose pathogenesis is not fully understood; however, it appears to represent a T-cell–mediated autoimmune disease. The prevailing theory is that exposure to an exogenous agent such as a virus, drug, or contact allergen alters epidermal self-antigens, leading to the activation of cytotoxic T cells. The altered self-antigens cross-react with normal self-antigens on basal keratinocytes, resulting in T-cell targeting and apoptosis.[6]
Various agents have been linked to the development of lichen planus, with particular emphasis on the association with viruses, especially the hepatitis C virus (HCV). Patients with lichen planus are 5 times more likely to test positive for HCV compared to the general population, and those with HCV seropositivity are 2.5 to 4.5 times more likely to develop lichen planus.[7][8] Recently, lichen planus has also been linked with the COVID-19 virus and its vaccine.[9]
Oral lichen planus is correlated with contact allergies to various metals found in dental restorations, including mercury, copper, and gold. Removal of the sensitizing metal has been reported to lead to the clearance of lichen planus lesions.[10][11]
A large number of drugs are associated with lichen planus; however, the recurrence of lesions after re-exposure to the drug is rare. More commonly associated drugs include antimalarials, angiotensin-converting enzyme inhibitors, thiazide diuretics, nonsteroidal anti-inflammatory drugs, quinidine, beta-blockers, tumor necrosis factor-α inhibitors, and gold.[5][12]
Epidemiology
The prevalence of cutaneous lichen planus is approximately 0.2% to 1% among adults worldwide.[13] Oral lichen planus is more common and reported in 1% to 4% of the population. Overall, women are more frequently affected compared to men at a ratio of 1.5:1, and most cases develop between the ages of 30 and 60. The condition is rare in children, representing less than 5% of all affected patients.[7] Although lichen planus is not generally considered to have an ethnic predilection, recent studies suggest a higher incidence of the disease in African Americans and individuals of Indian and Arabian descent.[14][15] There appears to be a familial component, as up to 10% of first-degree relatives of patients may also develop the disease.[16]
Pathophysiology
Multiple mechanisms explain the pathophysiology of lichen planus. Current understanding suggests that the immunopathogenesis of lichen planus is caused by cell-mediated cytotoxicity, particularly cytotoxic T lymphocytes, whose activity is further influenced by TH1 and the interleukin 23/TH17 axis. Other immunocytes and inflammatory pathways complement these mechanisms.[17]
Histopathology
Skin biopsy and microscopic analysis are valuable in confirming the diagnosis in atypical and severe cases, as the histopathologic features are generally the same regardless of the distribution or subtype. Principal findings include thickening of the stratum corneum without nuclei (hyperkeratosis without parakeratosis), irregular thickening of the stratum granulosum, destruction of the stratum basale, alteration or loss of rete ridges resulting in a sawtooth appearance, and a dense band of lymphocytes infiltrating the dermis along the dermo-epidermal junction (interface dermatitis). In addition, idiopathic lichen planus rarely demonstrates eosinophils, whereas drug-induced lichen planus may show eosinophils.
Apoptotic keratinocytes are often observed near the basal layer and are termed colloid or Civatte bodies. Direct immunofluorescence staining may display colloid bodies with irregular deposits of immunoglobulin A (IgA), IgM, IgG, or C3.[18][19]
History and Physical
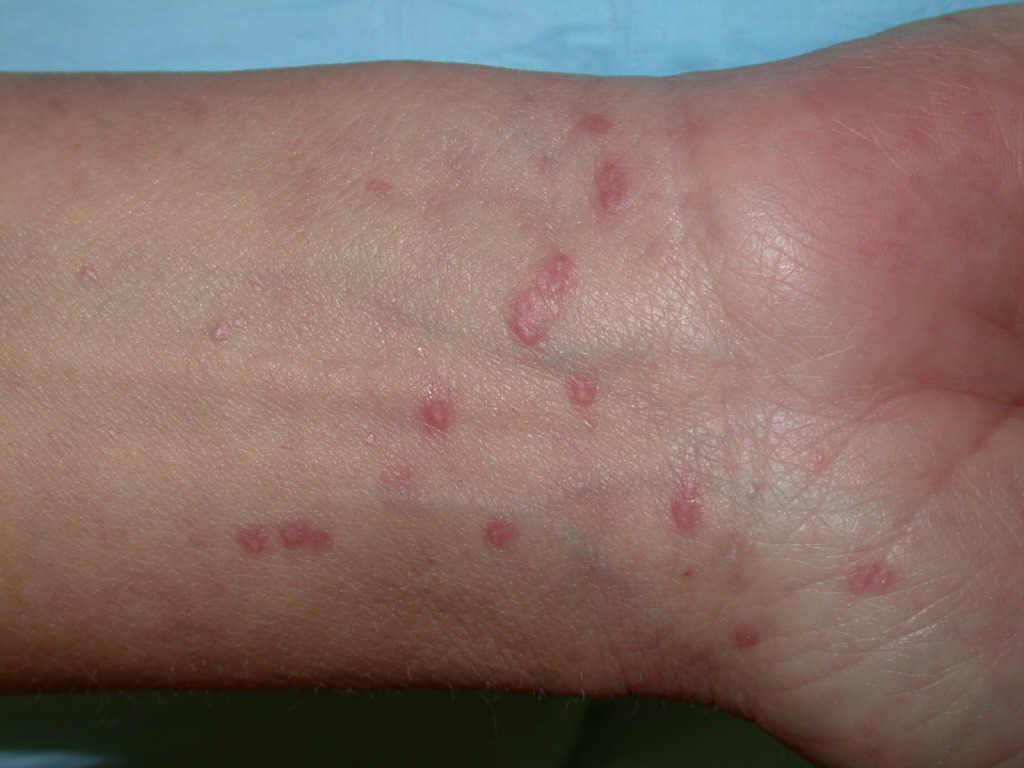
Lichen planus can display various lesion types, but the most common presentation is an area of polygon-shaped, itchy, violaceous, flat-topped papules a few millimeters wide. This classic presentation is known as the 6 Ps of lichen planus—purple, polygonal, planar, pruritic, papules, and plaques (see Image. Lichen Planus). The lesions have a shiny surface covered in fine white lines known as Wickham striae and are firm on palpation. These lesions may appear as a few individual lesions, scattered widely, grouped in plaques, or arranged in annular, linear, or actinic (sun-exposed) patterns. The isomorphic response, known as the Koebner phenomenon, can occur in lichen planus, wherein new lesions arise in lines where scratching occurs, similar to what is observed in psoriasis. The most common areas of involvement include the flexor wrists, dorsal hands, lower back, ankles, and shins. Frequently, grayish-brown hyperpigmentation can be found after lesions resolve due to the deposition of melanin in the superficial dermis.[7][13]
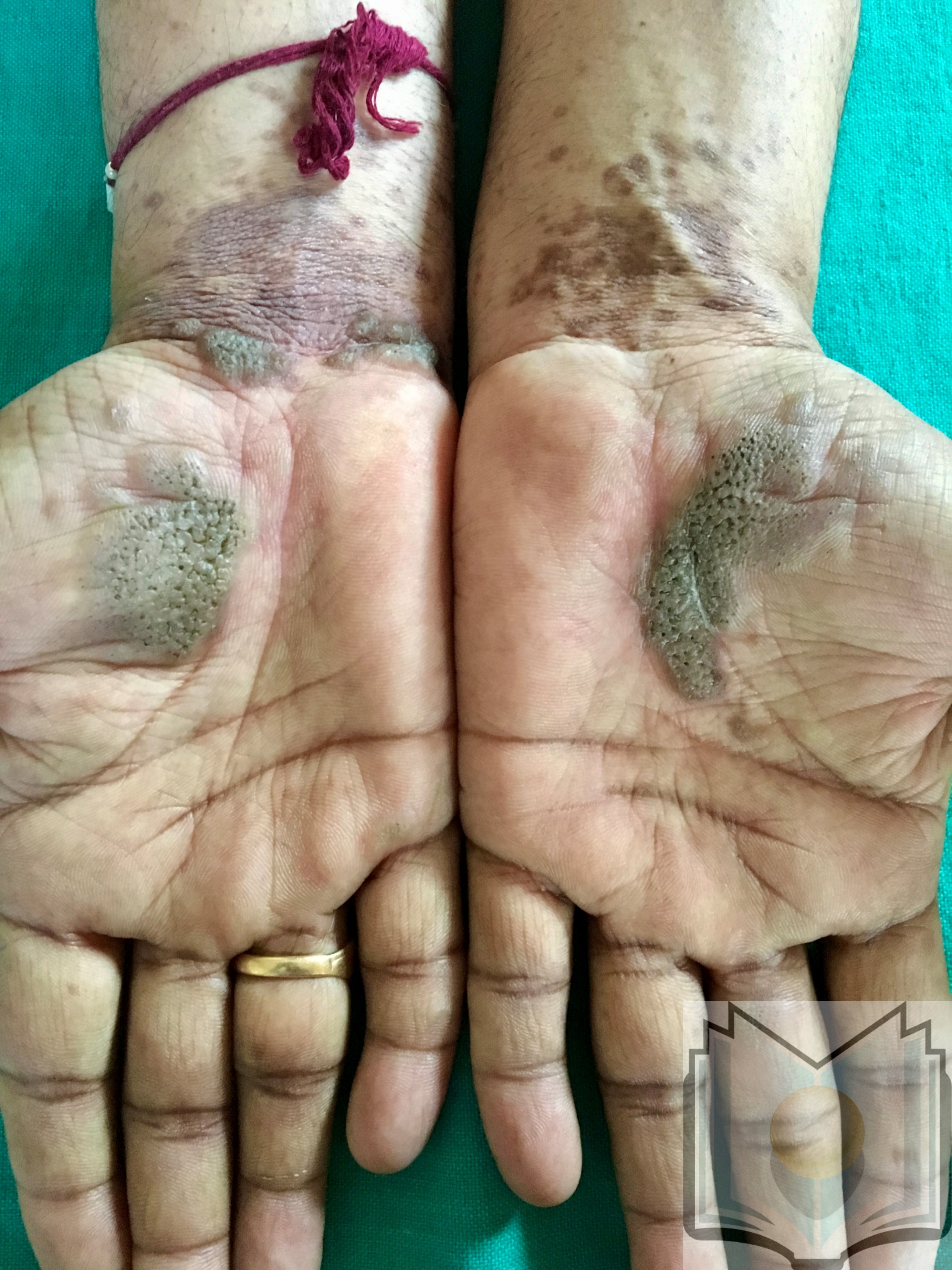
Various subtypes of lichen planus exist that display patterns different from the classic presentation. Hypertrophic lichen planus is often found on the shins and ankles and is characterized by red, red-brown, or yellow-grey papules and plaques that coalesce with a thickened or verrucous surface. Ulcerative lichen planus is found on the soles of the feet or between the toes, with painful, erosive lesions that complicate walking (see Image. Lichen Planus, Palms). Bullous lichen planus often appears on the legs as small to large tense blisters filled with clear or pale-yellow fluid. Lichen planus pemphigoids display features of lichen planus with the development of bullae both on top of lichen planus lesions and on unaltered skin. Lichen planus pigmentosus is characterized by the development of macular or papular pigmented lesions often arranged in a linear, follicular, or Blashkoid pattern. Inverse lichen planus is similar to inverse psoriasis as it is found in skin folds and loses its typical appearance. Extensive erythematous lesions with lichenification and without distinct borders are observed in the axillae, limb flexures, inguinal creases, and beneath the breasts.[20]
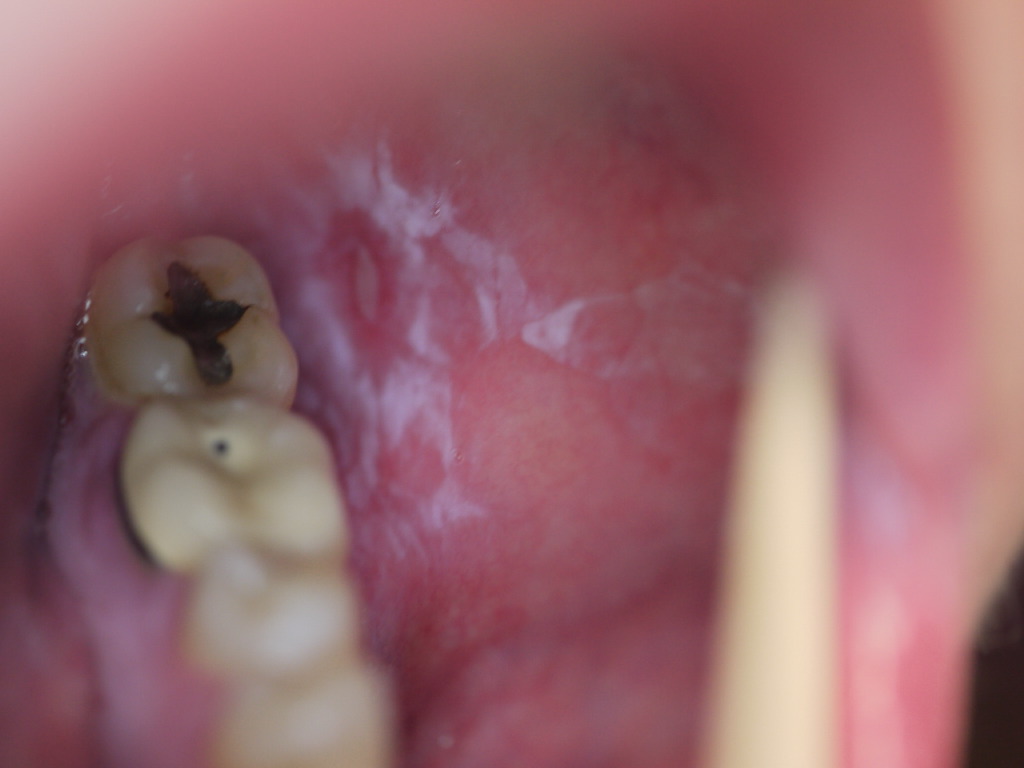
Mucosal involvement affects over half of all patients with lichen planus and frequently is the sole presenting sign, most commonly observed in the mouth, but it can also be found on the lips, esophagus, glans penis, vulva, or vagina. Six subtypes of oral lichen planus exist—reticular, erosive, papular, plaque-like, atrophic, and bullous. Reticular is the most common form and presents as asymptomatic white, lacy lines often observed on the bilateral buccal mucosa (see Image. Oral Lichen Planus). The erosive and atrophic forms are commonly associated with a burning pain exacerbated by hot or spicy foods. Lesions can easily be mistaken for leukoplakia or candidiasis on the tongue or buccal mucosa. Esophageal lichen planus predominantly affects women and can give rise to dysphagia, strictures, and possibly squamous cell carcinoma.[10][21][22]
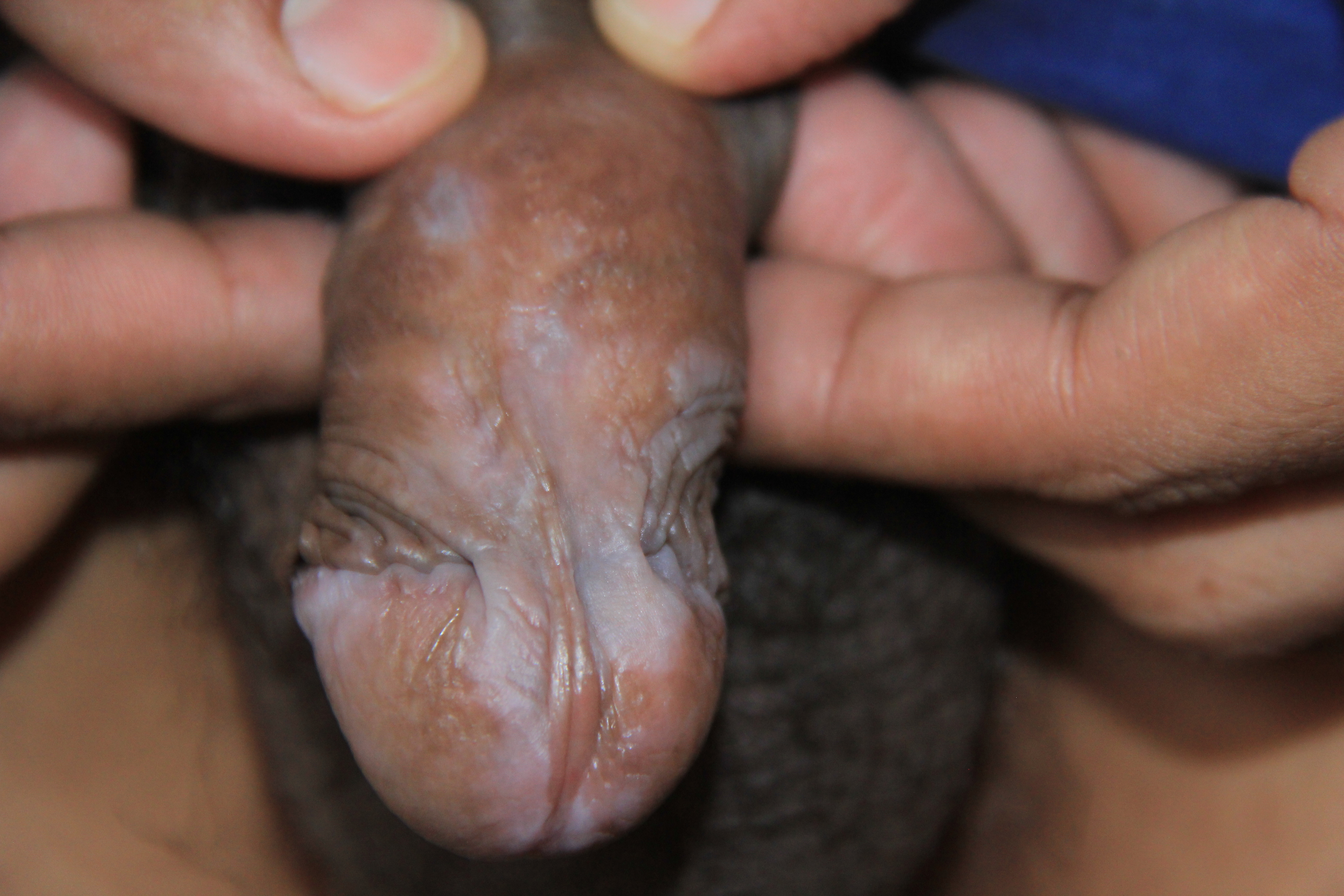
When lichen planus affects the glans penis, it commonly displays an annular configuration (see Image. Lichen Planus, Penis). In contrast, an erosive variant is most frequently found in women when lichen planus involves the vulva or vagina, and scarring and strictures are troublesome sequelae that may occur. Lichen planus found simultaneously on the female genital and oral mucosa is termed vulvovaginal-gingival syndrome. This severe, desquamative subtype has been associated with the HLA-DQB1*02:01 gene in 80% of patients, suggesting a genetic predilection for its development.[23][24]
Lichen planus of the nails occurs in approximately 10% of patients, typically affecting multiple nails without necessarily involving the surrounding skin.[25] Early signs include thinning of the nail plate and longitudinal ridging. Continued involvement leads to scarring of the nail matrix, dorsal pterygium formation, sandpaper nails (trachonychia), and possibly complete loss of the nail plate. Some patients may exhibit a variant of lichen planus called 20-nail dystrophy, where these findings are the only presenting sign of disease on all 20 nails. This subtype is much more common in children compared to adults.[26][27]
Lichen planus of the scalp and other hair-bearing regions is called lichen planopilaris (LPP). Small red follicular papules and macules appear at the site of inflammation, leading to progressive scarring alopecia. LPP may appear alone or with typical lichen planus lesions elsewhere on the body. When LPP occurs principally on the anterior scalp and eyebrows in older women, it is known as frontal fibrosing alopecia. A familial variant of LPP known as Graham-Little-Piccardi-Lasseur syndrome is characterized by scarring alopecia of the scalp, typical cutaneous or mucosal lichen planus, and nonscarring loss of pubic and axillary hairs with follicular papules.[28][29]
Lichenoid drug eruptions often appear in sun-exposed areas rather than appearing in the classic lichen planus sites. These eruptions are typically symmetric and are more generalized in distribution. Lesions may resemble eczematous or psoriasiform conditions, and Wickham striae are not commonly observed. There is typically a latent period of several months to a year from the initiation of a drug to an eruption of lesions. Hence, a thorough review of medication history is crucial for diagnosing drug-induced lichen planus.[5]
Evaluation
Evaluating lichen planus involves a comprehensive clinical assessment to accurately diagnose the condition and distinguish it from similar dermatological disorders. While in the clinic, dermoscopy allows visualization of Wickham striae in most cases. The classic finding is a network of white lines with red globules along the periphery.[30] Oral lichen planus lesions located near dental restorations should prompt patch testing to determine whether an allergy to one of the associated metals exists.[31]
A biopsy with microscopic analysis is the most effective tool for confirming the presence of lichen planus. The lesions display distinct features, as noted previously, that typically facilitate a definitive diagnosis. Incorporating direct immunofluorescence into histology can help differentiate lichen planus from other similar conditions, such as lupus erythematosus.[32]
Treatment / Management
Cutaneous lichen planus typically resolves spontaneously within 1 to 2 years; therefore, treatment is aimed at reducing pruritus and allowing time for resolution. For limited lichen planus, the recommended first-line treatment is superpotent topical steroids (clobetasol 0.05%) twice daily for 2 to 4 weeks. Inadequate response to topical steroids may be augmented with intralesional steroid injections (triamcinolone 5 to 10 mg/mL). For diffuse lichen planus, the first-line treatment is daily oral corticosteroids (prednisone 30 to 60 mg) tapered over 2 to 6 weeks.
If no change is observed, second-line therapy should be considered. Second-line therapy may include metronidazole (500 mg twice daily for 3 to 8 weeks), sulfasalazine (500 mg twice daily increased in 500 mg increments every 3 days until 2.5 g daily is reached, for 3 to 6 weeks), isotretinoin (10 mg twice daily for 2 months), acitretin (30 mg daily for 8 weeks), psoralen and ultraviolet A, ultraviolet B, topical calcineurin inhibitors, or methotrexate (15 mg per week for adults, 0.25 mg/kg per week for children). Third-line treatment may include trimethoprim-sulfamethoxazole, griseofulvin, terbinafine, antimalarials, tetracyclines, ciclosporin, mycophenolate mofetil, azathioprine, etanercept, adalimumab, or low-molecular-weight heparin.[7][33]
Oral lichen planus may spontaneously resolve within 5 years, but many cases are chronic and do not resolve. Treatment-induced remission is typically followed by relapse. Thus, asymptomatic oral lichen planus should not be treated due to the high burden of adverse effects associated with treatment. The goal of treating symptomatic oral lichen planus is to heal erosive lesions, reduce pain, and allow normal food intake. Patients should be instructed to avoid spicy or acidic foods, alcohol, and tobacco, as these exacerbate symptoms. First-line treatment involves applying a very high-potency topical steroid 3 times daily until remission. If there is no improvement after 6 weeks, treatment should be escalated. The second-line treatment includes oral corticosteroids or the application of topical calcineurin inhibitors. Third-line treatment may include cyclosporine, azathioprine, mycophenolate mofetil, or methotrexate.[10][34]
Consideration of drug-induced lichen planus must always be explored before starting therapy. Withdrawal of the suspected drug leading to the gradual disappearance of lesions confirms the diagnosis, although it may take some time for lesions to resolve fully.[35]
Differential Diagnosis
Given that lichen planus may result from various exogenous agents such as viruses, drugs, or contact allergens, care should be taken to identify and treat any underlying causes before diagnosing idiopathic lichen planus. Various diseases that may resemble lichen planus include lupus erythematosus, erythema dyschromicum perstans, psoriasis, secondary syphilis, pityriasis rosea, lichen nitidus, graft-versus-host disease, and keratosis lichenoides chronica. Hypertrophic lichen planus can appear very similar to lichen simplex chronicus. Vulvar lichen planus can be difficult to distinguish from lichen sclerosis.
Differentiating between lichen planus and lupus erythematosus is challenging when lesions are only present on the scalp or in the mouth; therefore, biopsy with direct immunofluorescence is especially useful in these cases. The simultaneous presence of both diseases has been described in many reports, possibly related to the use of antimalarials in treating lupus erythematosus.[32][36]
Prognosis
Cutaneous lichen planus often resolves spontaneously within 1 to 2 years, but residual hyperpigmentation is common. Oral lichen planus may resolve spontaneously within 5 years, but typically, it is a chronic disease with a remitting and relapsing course. Hair loss from LPP is permanent. Drug-induced lichen planus lesions resolve slowly after the causative medication is withdrawn.
Complications
Lichen planus can lead to several complications, especially when it becomes chronic or involves mucosal surfaces. The condition can cause painful erosive lesions in the oral cavity, increasing the risk of secondary infections and possibly malignant transformation into oral squamous cell carcinoma. Genital involvement may result in scarring and strictures, whereas skin lesions can cause post-inflammatory hyperpigmentation. Nail involvement can lead to permanent nail loss or dystrophy. In severe cases, patients may experience significant discomfort, affecting their quality of life. Long-term care should include careful monitoring for malignant transformation and effective symptom management.[37]
Consultations
Lichen planus may affect the skin, scalp, and mucosal organs and is relatively difficult to cure. Patients with lichen planus may benefit from consultations with various specialists to ensure comprehensive care. Dermatologists play a crucial role in managing cutaneous lesions, whereas oral health professionals, such as dentists or oral pathologists, are important for diagnosing and treating oral lichen planus. In cases of drug-induced lichen planus, collaboration with pharmacists is crucial to adjust medications and minimize triggers. In addition, referrals to specialists such as allergists or rheumatologists may be necessary for patients with complex cases or associated autoimmune conditions. These interdisciplinary consultations help tailor treatment plans, improving patient outcomes and disease management.
Deterrence and Patient Education
Deterrence and patient education are essential components in effectively managing lichen planus, significantly influencing patient outcomes and overall quality of life. A comprehensive understanding of the condition helps patients navigate their diagnosis more confidently. Education should begin with an overview of lichen planus, emphasizing that it is a chronic inflammatory disorder of the skin and mucous membranes with no known cause. By clarifying that the condition is not contagious but can be associated with various triggers, patients can better appreciate the importance of identifying and avoiding these factors.
Key elements of patient education include discussing potential triggers such as certain medications, allergies, stress, and skin trauma. Patients should be advised to maintain a detailed record of their symptoms and any lifestyle changes that coincide with flare-ups, enabling them to identify patterns that may indicate specific triggers. For instance, drug-induced lichen planus often occurs after exposure to certain medications; therefore, educating patients on the need to review their prescriptions with their healthcare professionals is vital.
Patients should be informed about the various forms of lichen planus, including its cutaneous and oral manifestations, and the differing prognoses and management strategies associated with each. This knowledge empowers patients to monitor their symptoms closely and seek timely medical advice if new lesions develop or existing ones worsen. The educational approach should include practical strategies for managing discomfort, such as the use of topical corticosteroids, antihistamines for itching, or oral medications for more severe cases.
In addition, reinforcing the importance of regular follow-up appointments is essential for ongoing assessment and management of lichen planus. These visits allow clinicians to monitor the condition, adjust treatment plans, and address patient concerns. Regular communication ensures that patients remain informed about their health status and are involved in shared decision-making regarding their treatment options.
Resources such as informational pamphlets, online materials, and support groups can further enhance patient education. Support groups allow patients to connect with others facing similar challenges, fostering a sense of community and shared experiences. Such interactions can alleviate feelings of isolation and provide practical tips for coping with the emotional and physical aspects of living with lichen planus.
Enhancing Healthcare Team Outcomes
Enhancing patient-centered care and outcomes for individuals with lichen planus involves a collaborative interprofessional approach. Healthcare professionals must develop strong clinical assessment skills to accurately diagnose and manage lichen planus. Physicians and advanced practitioners should be skilled in recognizing the characteristic presentations of lichen planus, whereas nurses play a crucial role in patient education and monitoring symptoms. Pharmacists contribute by managing medication regimens and counseling patients about potential drug-induced lichen planus.[35]
Coordinating care among physicians, nurses, pharmacists, and other health professionals is crucial for optimizing patient outcomes. This effort includes establishing clear referral pathways for specialists, such as dermatologists or oral healthcare professionals, when necessary. Care coordinators can help track appointments and follow-up treatments, ensuring patients have access to the resources and support they need.
Patient education about the condition, its management, and potential complications is vital, as is fostering an environment where patients feel comfortable discussing their concerns. Ongoing evaluation and adaptation of care plans based on patient feedback and clinical outcomes improve safety and team performance.