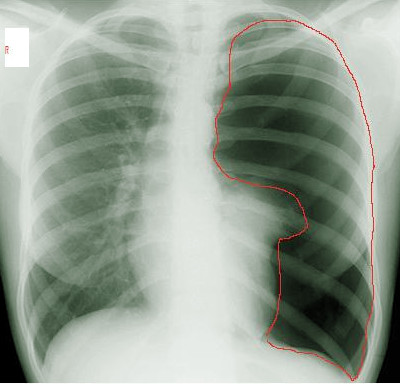
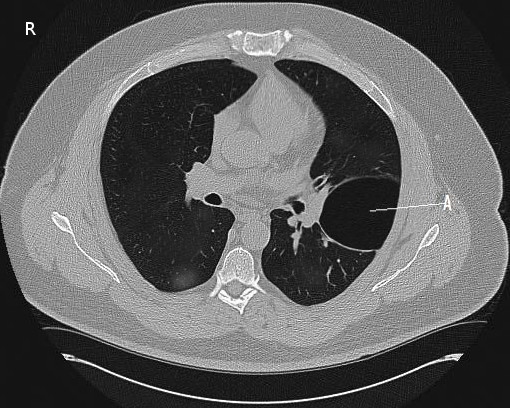
[1]
Loiselle A, Parish JM, Wilkens JA, Jaroszewski DE. Managing iatrogenic pneumothorax and chest tubes. Journal of hospital medicine. 2013 Jul:8(7):402-8. doi: 10.1002/jhm.2053. Epub 2013 Jun 14
[PubMed PMID: 23765922]
[2]
Garg SK, Garg P, Anchan N, Jaiswal A. Iatrogenic Bilateral Simultaneous Pneumothorax: Call for Vigilance. Indian journal of critical care medicine : peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2017 Sep:21(9):607-609. doi: 10.4103/ijccm.IJCCM_108_17. Epub
[PubMed PMID: 28970663]
[3]
Arteaga AA, Pitts KD, Lewis AF. Iatrogenic pneumothorax during hypoglossal nerve stimulator implantation. American journal of otolaryngology. 2018 Sep-Oct:39(5):636-638. doi: 10.1016/j.amjoto.2018.06.014. Epub 2018 Jun 14
[PubMed PMID: 29941192]
[4]
Tagami R, Moriya T, Kinoshita K, Tanjoh K. Bilateral tension pneumothorax related to acupuncture. Acupuncture in medicine : journal of the British Medical Acupuncture Society. 2013 Jun:31(2):242-4. doi: 10.1136/acupmed-2012-010284. Epub 2013 Feb 28
[PubMed PMID: 23449179]
[5]
Shieh L, Go M, Gessner D, Chen JH, Hopkins J, Maggio P. Improving and sustaining a reduction in iatrogenic pneumothorax through a multifaceted quality-improvement approach. Journal of hospital medicine. 2015 Sep:10(9):599-607. doi: 10.1002/jhm.2388. Epub 2015 Jun 3
[PubMed PMID: 26041246]
Level 2 (mid-level) evidence
[6]
Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. The Thoracic and cardiovascular surgeon. 2009 Aug:57(5):286-90. doi: 10.1055/s-0029-1185365. Epub 2009 Jul 23
[PubMed PMID: 19629891]
[7]
Kilbourne MJ, Bochicchio GV, Scalea T, Xiao Y. Avoiding common technical errors in subclavian central venous catheter placement. Journal of the American College of Surgeons. 2009 Jan:208(1):104-9. doi: 10.1016/j.jamcollsurg.2008.09.025. Epub
[PubMed PMID: 19228511]
[8]
John J, Seifi A. Incidence of iatrogenic pneumothorax in the United States in teaching vs. non-teaching hospitals from 2000 to 2012. Journal of critical care. 2016 Aug:34():66-8. doi: 10.1016/j.jcrc.2016.03.013. Epub 2016 Mar 31
[PubMed PMID: 27288612]
[9]
Swierzy M, Helmig M, Ismail M, Rückert J, Walles T, Neudecker J. [Pneumothorax]. Zentralblatt fur Chirurgie. 2014 Sep:139 Suppl 1():S69-86; quiz S87. doi: 10.1055/s-0034-1383029. Epub 2014 Sep 29
[PubMed PMID: 25264729]
[10]
Haynes D, Baumann MH. Management of pneumothorax. Seminars in respiratory and critical care medicine. 2010 Dec:31(6):769-80. doi: 10.1055/s-0030-1269837. Epub 2011 Jan 6
[PubMed PMID: 21213209]
[11]
Hefny AF, Kunhivalappil FT, Matev N, Avila NA, Bashir MO, Abu-Zidan FM. Management of computed tomography-detected pneumothorax in patients with blunt trauma: experience from a community-based hospital. Singapore medical journal. 2018 Mar:59(3):150-154. doi: 10.11622/smedj.2017074. Epub 2017 Jul 25
[PubMed PMID: 28741012]
[12]
Tupchong K. Update: Is Needle Aspiration Better Than Chest Tube Placement for the Management of Primary Spontaneous Pneumothorax? Annals of emergency medicine. 2018 Jul:72(1):e1-e2. doi: 10.1016/j.annemergmed.2018.02.025. Epub 2018 Apr 1
[PubMed PMID: 29615265]
[13]
Bruschettini M, Romantsik O, Ramenghi LA, Zappettini S, O'Donnell CP, Calevo MG. Needle aspiration versus intercostal tube drainage for pneumothorax in the newborn. The Cochrane database of systematic reviews. 2016 Jan 11:(1):CD011724. doi: 10.1002/14651858.CD011724.pub2. Epub 2016 Jan 11
[PubMed PMID: 26751585]
Level 1 (high-level) evidence
[14]
Naik ND, Hernandez MC, Anderson JR, Ross EK, Zielinski MD, Aho JM. Needle Decompression of Tension Pneumothorax with Colorimetric Capnography. Chest. 2017 Nov:152(5):1015-1020. doi: 10.1016/j.chest.2017.04.179. Epub 2017 May 10
[PubMed PMID: 28499514]
[15]
Wang C, Lyu M, Zhou J, Liu Y, Ji Y. Chest tube drainage versus needle aspiration for primary spontaneous pneumothorax: which is better? Journal of thoracic disease. 2017 Oct:9(10):4027-4038. doi: 10.21037/jtd.2017.08.140. Epub
[PubMed PMID: 29268413]
[16]
Drinhaus H, Annecke T, Hinkelbein J. [Chest decompression in emergency medicine and intensive care]. Der Anaesthesist. 2016 Oct:65(10):768-775
[PubMed PMID: 27629501]
[17]
Delpy JP, Pagès PB, Mordant P, Falcoz PE, Thomas P, Le Pimpec-Barthes F, Dahan M, Bernard A, EPITHOR project (French Society of Thoracic and Cardiovascular Surgery), EPITHOR project French Society of Thoracic and Cardiovascular Surgery. Surgical management of spontaneous pneumothorax: are there any prognostic factors influencing postoperative complications? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2016 Mar:49(3):862-7. doi: 10.1093/ejcts/ezv195. Epub 2015 Jun 12
[PubMed PMID: 26071433]
[18]
Tsotsolis N, Tsirgogianni K, Kioumis I, Pitsiou G, Baka S, Papaiwannou A, Karavergou A, Rapti A, Trakada G, Katsikogiannis N, Tsakiridis K, Karapantzos I, Karapantzou C, Barbetakis N, Zissimopoulos A, Kuhajda I, Andjelkovic D, Zarogoulidis K, Zarogoulidis P. Pneumothorax as a complication of central venous catheter insertion. Annals of translational medicine. 2015 Mar:3(3):40. doi: 10.3978/j.issn.2305-5839.2015.02.11. Epub
[PubMed PMID: 25815301]
[19]
Barsuk JH, Cohen ER, Williams MV, Scher J, Jones SF, Feinglass J, McGaghie WC, O'Hara K, Wayne DB. Simulation-Based Mastery Learning for Thoracentesis Skills Improves Patient Outcomes: A Randomized Trial. Academic medicine : journal of the Association of American Medical Colleges. 2018 May:93(5):729-735. doi: 10.1097/ACM.0000000000001965. Epub
[PubMed PMID: 29068818]
Level 1 (high-level) evidence
[20]
Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, Luketich JD, Panacek EA, Sahn SA, AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001 Feb:119(2):590-602
[PubMed PMID: 11171742]
Level 3 (low-level) evidence
[21]
Zonies D, Elterman J, Burns C, Paul V, Oh J, Cannon J. Trauma patients are safe to fly 72 hours after tube thoracostomy removal. The journal of trauma and acute care surgery. 2018 Sep:85(3):491-494. doi: 10.1097/TA.0000000000001976. Epub
[PubMed PMID: 29782482]
[22]
Roberts DJ, Leigh-Smith S, Faris PD, Blackmore C, Ball CG, Robertson HL, Dixon E, James MT, Kirkpatrick AW, Kortbeek JB, Stelfox HT. Clinical Presentation of Patients With Tension Pneumothorax: A Systematic Review. Annals of surgery. 2015 Jun:261(6):1068-78. doi: 10.1097/SLA.0000000000001073. Epub
[PubMed PMID: 25563887]
Level 1 (high-level) evidence
[23]
Slade M. Management of pneumothorax and prolonged air leak. Seminars in respiratory and critical care medicine. 2014 Dec:35(6):706-14. doi: 10.1055/s-0034-1395502. Epub 2014 Dec 2
[PubMed PMID: 25463161]
[25]
Giacomini M, Iapichino G, Armani S, Cozzolino M, Brancaccio D, Gallieni M. How to avoid and manage a pneumothorax. The journal of vascular access. 2006 Jan-Mar:7(1):7-14
[PubMed PMID: 16596523]
[26]
Soffler MI, Hayes MM, Smith CC. Central venous catheterization training: current perspectives on the role of simulation. Advances in medical education and practice. 2018:9():395-403. doi: 10.2147/AMEP.S142605. Epub 2018 May 25
[PubMed PMID: 29872360]
Level 3 (low-level) evidence
[27]
Ma IW, Brindle ME, Ronksley PE, Lorenzetti DL, Sauve RS, Ghali WA. Use of simulation-based education to improve outcomes of central venous catheterization: a systematic review and meta-analysis. Academic medicine : journal of the Association of American Medical Colleges. 2011 Sep:86(9):1137-47. doi: 10.1097/ACM.0b013e318226a204. Epub
[PubMed PMID: 21785310]
Level 1 (high-level) evidence
[28]
Sekiguchi H, Tokita JE, Minami T, Eisen LA, Mayo PH, Narasimhan M. A prerotational, simulation-based workshop improves the safety of central venous catheter insertion: results of a successful internal medicine house staff training program. Chest. 2011 Sep:140(3):652-658. doi: 10.1378/chest.10-3319. Epub 2011 Jun 9
[PubMed PMID: 21659429]