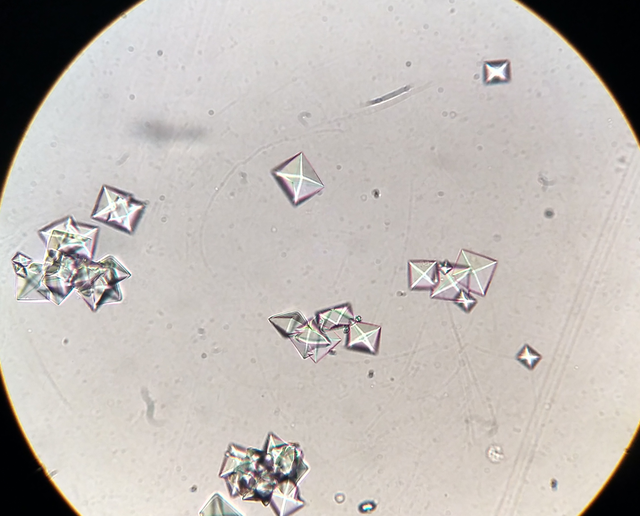
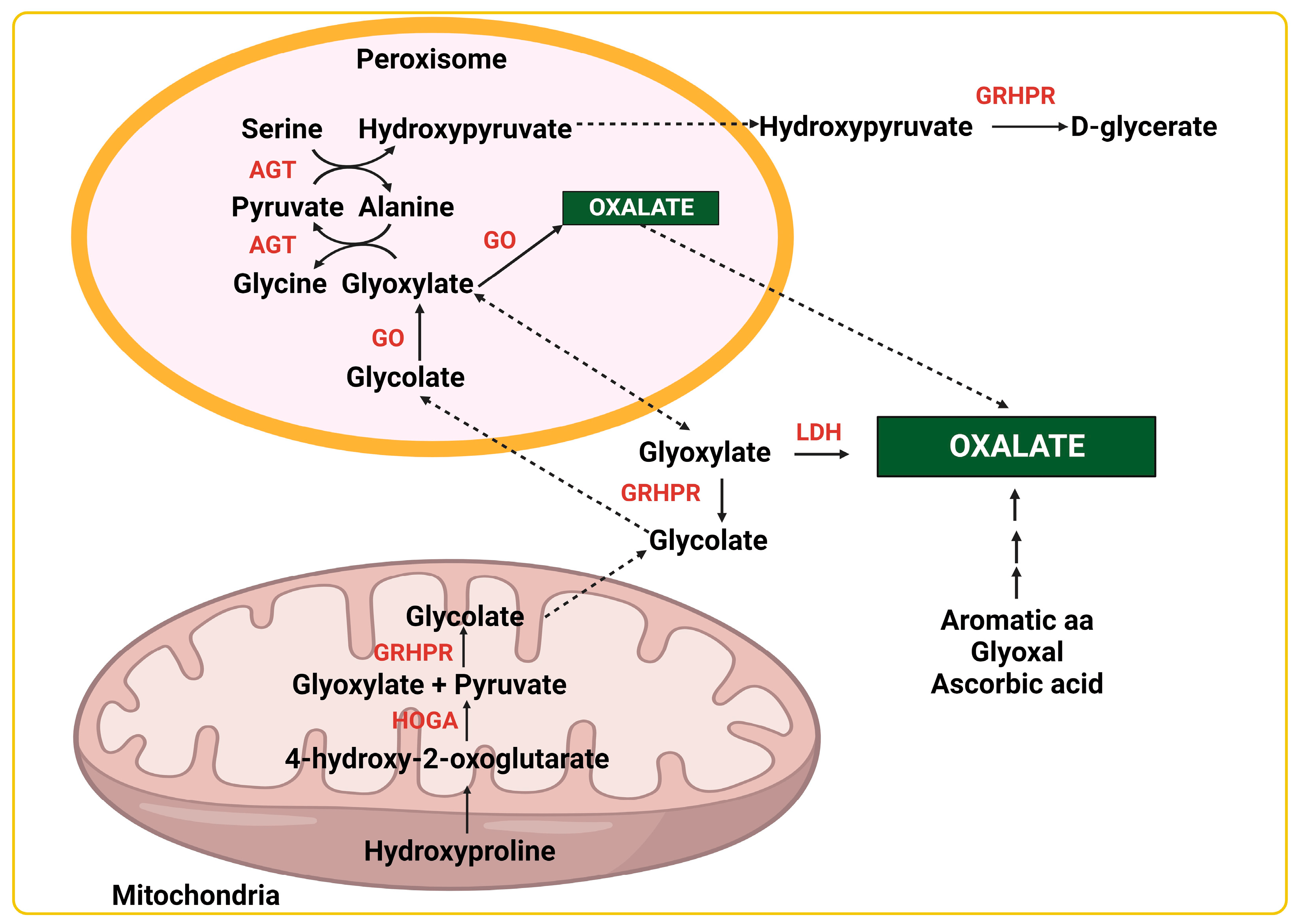
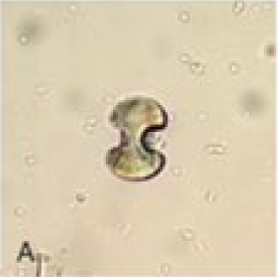
[2]
Bhasin B, Ürekli HM, Atta MG. Primary and secondary hyperoxaluria: Understanding the enigma. World journal of nephrology. 2015 May 6:4(2):235-44. doi: 10.5527/wjn.v4.i2.235. Epub
[PubMed PMID: 25949937]
Level 3 (low-level) evidence
[5]
Taylor EN, Curhan GC. Determinants of 24-hour urinary oxalate excretion. Clinical journal of the American Society of Nephrology : CJASN. 2008 Sep:3(5):1453-60. doi: 10.2215/CJN.01410308. Epub 2008 Jul 23
[PubMed PMID: 18650406]
[6]
Buysschaert B, Aydin S, Morelle J, Gillion V, Jadoul M, Demoulin N. Etiologies, Clinical Features, and Outcome of Oxalate Nephropathy. Kidney international reports. 2020 Sep:5(9):1503-1509. doi: 10.1016/j.ekir.2020.06.021. Epub 2020 Jul 2
[PubMed PMID: 32954074]
[7]
Bao D, Wang Y, Zhao MH. Oxalate Nephropathy and the Mechanism of Oxalate-Induced Kidney Injury. Kidney diseases (Basel, Switzerland). 2023 Dec:9(6):459-468. doi: 10.1159/000533295. Epub 2023 Jul 27
[PubMed PMID: 38089442]
[8]
Shee K, Stoller ML. Perspectives in primary hyperoxaluria - historical, current and future clinical interventions. Nature reviews. Urology. 2022 Mar:19(3):137-146. doi: 10.1038/s41585-021-00543-4. Epub 2021 Dec 8
[PubMed PMID: 34880452]
Level 3 (low-level) evidence
[9]
Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kidney international. 2001 Jan:59(1):270-6
[PubMed PMID: 11135080]
[10]
Mitchell T, Kumar P, Reddy T, Wood KD, Knight J, Assimos DG, Holmes RP. Dietary oxalate and kidney stone formation. American journal of physiology. Renal physiology. 2019 Mar 1:316(3):F409-F413. doi: 10.1152/ajprenal.00373.2018. Epub 2018 Dec 19
[PubMed PMID: 30566003]
[11]
Terris MK, Issa MM, Tacker JR. Dietary supplementation with cranberry concentrate tablets may increase the risk of nephrolithiasis. Urology. 2001 Jan:57(1):26-9
[PubMed PMID: 11164137]
[12]
Siener R, Bade DJ, Hesse A, Hoppe B. Dietary hyperoxaluria is not reduced by treatment with lactic acid bacteria. Journal of translational medicine. 2013 Dec 12:11():306. doi: 10.1186/1479-5876-11-306. Epub 2013 Dec 12
[PubMed PMID: 24330782]
[13]
Nazzal L, Puri S, Goldfarb DS. Enteric hyperoxaluria: an important cause of end-stage kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2016 Mar:31(3):375-82. doi: 10.1093/ndt/gfv005. Epub 2015 Feb 20
[PubMed PMID: 25701816]
[14]
Sidhu H, Schmidt ME, Cornelius JG, Thamilselvan S, Khan SR, Hesse A, Peck AB. Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract-dwelling bacterium Oxalobacter formigenes: possible prevention by gut recolonization or enzyme replacement therapy. Journal of the American Society of Nephrology : JASN. 1999 Nov:10 Suppl 14():S334-40
[PubMed PMID: 10541258]
[15]
Troxel SA, Sidhu H, Kaul P, Low RK. Intestinal Oxalobacter formigenes colonization in calcium oxalate stone formers and its relation to urinary oxalate. Journal of endourology. 2003 Apr:17(3):173-6
[PubMed PMID: 12803990]
[16]
Siener R, Ebert D, Hesse A. Urinary oxalate excretion in female calcium oxalate stone formers with and without a history of recurrent urinary tract infections. Urological research. 2001 Aug:29(4):245-8
[PubMed PMID: 11585279]
[17]
Sidhu H, Hoppe B, Hesse A, Tenbrock K, Brömme S, Rietschel E, Peck AB. Absence of Oxalobacter formigenes in cystic fibrosis patients: a risk factor for hyperoxaluria. Lancet (London, England). 1998 Sep 26:352(9133):1026-9
[PubMed PMID: 9759746]
[18]
Demoulin N, Issa Z, Crott R, Morelle J, Danse E, Wallemacq P, Jadoul M, Deprez PH. Enteric hyperoxaluria in chronic pancreatitis. Medicine. 2017 May:96(19):e6758. doi: 10.1097/MD.0000000000006758. Epub
[PubMed PMID: 28489752]
[19]
de Martines DGL, Gianotten S, F M Wetzels J, G van der Meijden WA. Secondary hyperoxaluria due to pancreatic insufficiency. The Netherlands journal of medicine. 2019 Oct:77(8):287-292
[PubMed PMID: 31814577]
[20]
Ghannoum M, Gosselin S, Hoffman RS, Lavergne V, Mégarbane B, Hassanian-Moghaddam H, Rif M, Kallab S, Bird S, Wood DM, Roberts DM, EXTRIP Workgroup. Extracorporeal treatment for ethylene glycol poisoning: systematic review and recommendations from the EXTRIP workgroup. Critical care (London, England). 2023 Feb 10:27(1):56. doi: 10.1186/s13054-022-04227-2. Epub 2023 Feb 10
[PubMed PMID: 36765419]
Level 1 (high-level) evidence
[21]
Bnaya A, Abu-Amer N, Beckerman P, Volkov A, Cohen-Hagai K, Greenberg M, Ben-Chetrit S, Ben Tikva Kagan K, Goldman S, Navarro HA, Sneineh MA, Rozen-Zvi B, Borovitz Y, Tobar A, Yanay NB, Biton R, Angel-Korman A, Rappoport V, Leiba A, Bathish Y, Farber E, Kaidar-Ronat M, Schreiber L, Shashar M, Kazarski R, Chernin G, Itzkowitz E, Atrash J, Iaina NL, Efrati S, Nizri E, Lurie Y, Ben Itzhak O, Assady S, Kenig-Kozlovsky Y, Shavit L. Acute Kidney Injury and Hair-Straightening Products: A Case Series. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2023 Jul:82(1):43-52.e1. doi: 10.1053/j.ajkd.2022.11.016. Epub 2023 Jan 5
[PubMed PMID: 36610611]
Level 2 (mid-level) evidence
[22]
Abu-Amer N, Silberstein N, Kunin M, Mini S, Beckerman P. Acute Kidney Injury following Exposure to Formaldehyde-Free Hair-Straightening Products. Case reports in nephrology and dialysis. 2022 May-Aug:12(2):112-116. doi: 10.1159/000525567. Epub 2022 Jul 11
[PubMed PMID: 36160636]
Level 3 (low-level) evidence
[23]
Viljoen A, Chaudhry R, Bycroft J. Renal stones. Annals of clinical biochemistry. 2019 Jan:56(1):15-27. doi: 10.1177/0004563218781672. Epub 2018 Jun 13
[PubMed PMID: 29792045]
[24]
Worcester EM, Coe FL. Nephrolithiasis. Primary care. 2008 Jun:35(2):369-91, vii. doi: 10.1016/j.pop.2008.01.005. Epub
[PubMed PMID: 18486720]
[25]
Han H, Segal AM, Seifter JL, Dwyer JT. Nutritional Management of Kidney Stones (Nephrolithiasis). Clinical nutrition research. 2015 Jul:4(3):137-52. doi: 10.7762/cnr.2015.4.3.137. Epub 2015 Jul 31
[PubMed PMID: 26251832]
[26]
Spradling K, Vernez SL, Khoyliar C, Morgan JB, Okhunov Z, Preminger GM, Lipkin ME, Landman J, Youssef RF. Prevalence of Hyperoxaluria in Urinary Stone Formers: Chronological and Geographical Trends and a Literature Review. Journal of endourology. 2016 Apr:30(4):469-75. doi: 10.1089/end.2015.0676. Epub 2016 Feb 9
[PubMed PMID: 26738689]
[27]
Powell CR, Stoller ML, Schwartz BF, Kane C, Gentle DL, Bruce JE, Leslie SW. Impact of body weight on urinary electrolytes in urinary stone formers. Urology. 2000 Jun:55(6):825-30
[PubMed PMID: 10840085]
[28]
Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney international. 2001 Jun:59(6):2290-8
[PubMed PMID: 11380833]
[29]
Lewandowski S, Rodgers A, Schloss I. The influence of a high-oxalate/low-calcium diet on calcium oxalate renal stone risk factors in non-stone-forming black and white South African subjects. BJU international. 2001 Mar:87(4):307-11
[PubMed PMID: 11251520]
[30]
Rodgers AL, Lewandowski S. Effects of 5 different diets on urinary risk factors for calcium oxalate kidney stone formation: evidence of different renal handling mechanisms in different race groups. The Journal of urology. 2002 Sep:168(3):931-6
[PubMed PMID: 12187193]
[31]
Goldfarb DS, Parks JH, Coe FL. Renal stone disease in older adults. Clinics in geriatric medicine. 1998 May:14(2):367-81
[PubMed PMID: 9536110]
[32]
Hopp K, Cogal AG, Bergstralh EJ, Seide BM, Olson JB, Meek AM, Lieske JC, Milliner DS, Harris PC, Rare Kidney Stone Consortium. Phenotype-Genotype Correlations and Estimated Carrier Frequencies of Primary Hyperoxaluria. Journal of the American Society of Nephrology : JASN. 2015 Oct:26(10):2559-70. doi: 10.1681/ASN.2014070698. Epub 2015 Feb 2
[PubMed PMID: 25644115]
[33]
Harambat J, Fargue S, Bacchetta J, Acquaviva C, Cochat P. Primary hyperoxaluria. International journal of nephrology. 2011:2011():864580. doi: 10.4061/2011/864580. Epub 2011 Jun 16
[PubMed PMID: 21748001]
[34]
Cochat P, Rumsby G. Primary hyperoxaluria. The New England journal of medicine. 2013 Aug 15:369(7):649-58. doi: 10.1056/NEJMra1301564. Epub
[PubMed PMID: 23944302]
[35]
Soliman NA, Mabrouk S. Primary hyperoxaluria type 1 in developing countries: novel challenges in a new therapeutic era. Clinical kidney journal. 2022 May:15(Suppl 1):i33-i36. doi: 10.1093/ckj/sfab203. Epub 2022 May 17
[PubMed PMID: 35592622]
[36]
Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, Traxer O, Tiselius HG. Kidney stones. Nature reviews. Disease primers. 2016 Feb 25:2():16008. doi: 10.1038/nrdp.2016.8. Epub 2016 Feb 25
[PubMed PMID: 27188687]
[37]
Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, Milliner DS, Harris PC, Sas DJ, Cogal AG, Lieske JC. Primary Hyperoxaluria Type 1. GeneReviews(®). 1993:():
[PubMed PMID: 20301460]
[38]
Waikar SS, Srivastava A, Palsson R, Shafi T, Hsu CY, Sharma K, Lash JP, Chen J, He J, Lieske J, Xie D, Zhang X, Feldman HI, Curhan GC, Chronic Renal Insufficiency Cohort study investigators. Association of Urinary Oxalate Excretion With the Risk of Chronic Kidney Disease Progression. JAMA internal medicine. 2019 Apr 1:179(4):542-551. doi: 10.1001/jamainternmed.2018.7980. Epub
[PubMed PMID: 30830167]
[39]
Ermer T, Eckardt KU, Aronson PS, Knauf F. Oxalate, inflammasome, and progression of kidney disease. Current opinion in nephrology and hypertension. 2016 Jul:25(4):363-71. doi: 10.1097/MNH.0000000000000229. Epub
[PubMed PMID: 27191349]
Level 3 (low-level) evidence
[40]
Leumann E, Hoppe B. The primary hyperoxalurias. Journal of the American Society of Nephrology : JASN. 2001 Sep:12(9):1986-1993. doi: 10.1681/ASN.V1291986. Epub
[PubMed PMID: 11518794]
[41]
Alelign T, Petros B. Kidney Stone Disease: An Update on Current Concepts. Advances in urology. 2018:2018():3068365. doi: 10.1155/2018/3068365. Epub 2018 Feb 4
[PubMed PMID: 29515627]
Level 3 (low-level) evidence
[43]
Leslie SW, Sajjad H, Bashir K. 24-Hour Urine Testing for Nephrolithiasis: Interpretation and Treatment Guidelines. StatPearls. 2024 Jan:():
[PubMed PMID: 29494055]
[44]
Hoppe B. An update on primary hyperoxaluria. Nature reviews. Nephrology. 2012 Jun 12:8(8):467-75. doi: 10.1038/nrneph.2012.113. Epub 2012 Jun 12
[PubMed PMID: 22688746]
[45]
Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, Milliner DS, Harris PC, Sas DJ, Lieske JC. Primary Hyperoxaluria Type 3. GeneReviews(®). 1993:():
[PubMed PMID: 26401545]
[46]
Cochat P, Hulton SA, Acquaviva C, Danpure CJ, Daudon M, De Marchi M, Fargue S, Groothoff J, Harambat J, Hoppe B, Jamieson NV, Kemper MJ, Mandrile G, Marangella M, Picca S, Rumsby G, Salido E, Straub M, van Woerden CS, OxalEurope. Primary hyperoxaluria Type 1: indications for screening and guidance for diagnosis and treatment. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012 May:27(5):1729-36. doi: 10.1093/ndt/gfs078. Epub
[PubMed PMID: 22547750]
[47]
Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, Monga M, Penniston KL, Preminger GM, Turk TM, White JR, American Urological Assocation. Medical management of kidney stones: AUA guideline. The Journal of urology. 2014 Aug:192(2):316-24. doi: 10.1016/j.juro.2014.05.006. Epub 2014 May 20
[PubMed PMID: 24857648]
[48]
Finkielstein VA, Goldfarb DS. Strategies for preventing calcium oxalate stones. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2006 May 9:174(10):1407-9
[PubMed PMID: 16682705]
[50]
Milliner DS, Eickholt JT, Bergstralh EJ, Wilson DM, Smith LH. Results of long-term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. The New England journal of medicine. 1994 Dec 8:331(23):1553-8
[PubMed PMID: 7969325]
[51]
Park S, Pearle MS. Pathophysiology and management of calcium stones. The Urologic clinics of North America. 2007 Aug:34(3):323-34
[PubMed PMID: 17678983]
[52]
Noonan SC, Savage GP. Oxalate content of foods and its effect on humans. Asia Pacific journal of clinical nutrition. 1999 Mar:8(1):64-74
[PubMed PMID: 24393738]
[53]
Dejban P, Lieske JC. New therapeutics for primary hyperoxaluria type 1. Current opinion in nephrology and hypertension. 2022 Jul 1:31(4):344-350. doi: 10.1097/MNH.0000000000000790. Epub 2022 Mar 9
[PubMed PMID: 35266883]
Level 3 (low-level) evidence
[54]
Hoppe B, Martin-Higueras C. Improving Treatment Options for Primary Hyperoxaluria. Drugs. 2022 Jul:82(10):1077-1094. doi: 10.1007/s40265-022-01735-x. Epub 2022 Jul 2
[PubMed PMID: 35779234]
[55]
Hoppe B, Groothoff JW, Hulton SA, Cochat P, Niaudet P, Kemper MJ, Deschênes G, Unwin R, Milliner D. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011 Nov:26(11):3609-15. doi: 10.1093/ndt/gfr107. Epub 2011 Apr 2
[PubMed PMID: 21460356]
[56]
Hoppe B, Niaudet P, Salomon R, Harambat J, Hulton SA, Van't Hoff W, Moochhala SH, Deschênes G, Lindner E, Sjögren A, Cochat P. A randomised Phase I/II trial to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Pediatric nephrology (Berlin, Germany). 2017 May:32(5):781-790. doi: 10.1007/s00467-016-3553-8. Epub 2016 Dec 6
[PubMed PMID: 27924398]
Level 1 (high-level) evidence
[57]
Milliner D, Hoppe B, Groothoff J. A randomised Phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis. 2018 Aug:46(4):313-323. doi: 10.1007/s00240-017-0998-6. Epub 2017 Jul 17
[PubMed PMID: 28718073]
Level 1 (high-level) evidence
[58]
Quintero E, Bird VY, Liu H, Stevens G, Ryan AS, Buzzerd S, Klimberg IW. A Prospective, Double-Blind, Randomized, Placebo-Controlled, Crossover Study Using an Orally Administered Oxalate Decarboxylase (OxDC). Kidney360. 2020 Nov 25:1(11):1284-1290. doi: 10.34067/KID.0001522020. Epub 2020 Sep 3
[PubMed PMID: 35372879]
Level 1 (high-level) evidence
[59]
Senthil D, Malini MM, Varalakshmi P. Sodium pentosan polysulphate--a novel inhibitor of urinary risk factors and enzymes in experimental urolithiatic rats. Renal failure. 1998 Jul:20(4):573-80
[PubMed PMID: 9713875]
[60]
Senthil D, Subha K, Saravanan N, Varalakshmi P. Influence of sodium pentosan polysulphate and certain inhibitors on calcium oxalate crystal growth. Molecular and cellular biochemistry. 1996 Mar 9:156(1):31-5
[PubMed PMID: 8709973]
[61]
Fellström B, Backman U, Danielson B, Wikström B. Treatment of renal calcium stone disease with the synthetic glycosaminoglycan pentosan polysulphate. World journal of urology. 1994:12(1):52-4
[PubMed PMID: 7516780]
[62]
Nakatani T, Ishii K, Yoneda Y, Kamikawa S, Kanazawa T, Sugimoto T, Osswald H. The preventive effect of sodium pentosan polysulfate against renal stone formation in hyperoxaluric rats. Urological research. 2002 Oct:30(5):329-35
[PubMed PMID: 12389123]
[63]
Erturk E, Kiernan M, Schoen SR. Clinical association with urinary glycosaminoglycans and urolithiasis. Urology. 2002 Apr:59(4):495-9
[PubMed PMID: 11927298]
[65]
Takei K, Ito H, Masai M, Kotake T. Oral calcium supplement decreases urinary oxalate excretion in patients with enteric hyperoxaluria. Urologia internationalis. 1998:61(3):192-5
[PubMed PMID: 9933846]
[66]
Damasio PC, Amaro CR, Cunha NB, Pichutte AC, Goldberg J, Padovani CR, Amaro JL. The role of salt abuse on risk for hypercalciuria. Nutrition journal. 2011 Jan 6:10():3. doi: 10.1186/1475-2891-10-3. Epub 2011 Jan 6
[PubMed PMID: 21211048]
[67]
Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002 Aug:40(2):265-74
[PubMed PMID: 12148098]
[68]
Nguyen QV, Kälin A, Drouve U, Casez JP, Jaeger P. Sensitivity to meat protein intake and hyperoxaluria in idiopathic calcium stone formers. Kidney international. 2001 Jun:59(6):2273-81
[PubMed PMID: 11380831]
Level 3 (low-level) evidence
[69]
Eusufzai S. Bile acid malabsorption: mechanisms and treatment. Digestive diseases (Basel, Switzerland). 1995 Sep-Oct:13(5):312-21
[PubMed PMID: 8542666]
[70]
Scott LJ, Keam SJ. Lumasiran: First Approval. Drugs. 2021 Feb:81(2):277-282. doi: 10.1007/s40265-020-01463-0. Epub
[PubMed PMID: 33405070]
[71]
Moya-Garzon MD, Gomez-Vidal JA, Alejo-Armijo A, Altarejos J, Rodriguez-Madoz JR, Fernandes MX, Salido E, Salido S, Diaz-Gavilan M. Small Molecule-Based Enzyme Inhibitors in the Treatment of Primary Hyperoxalurias. Journal of personalized medicine. 2021 Jan 27:11(2):. doi: 10.3390/jpm11020074. Epub 2021 Jan 27
[PubMed PMID: 33513899]
[72]
Garrelfs SF, Frishberg Y, Hulton SA, Koren MJ, O'Riordan WD, Cochat P, Deschênes G, Shasha-Lavsky H, Saland JM, Van't Hoff WG, Fuster DG, Magen D, Moochhala SH, Schalk G, Simkova E, Groothoff JW, Sas DJ, Meliambro KA, Lu J, Sweetser MT, Garg PP, Vaishnaw AK, Gansner JM, McGregor TL, Lieske JC, ILLUMINATE-A Collaborators. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. The New England journal of medicine. 2021 Apr 1:384(13):1216-1226. doi: 10.1056/NEJMoa2021712. Epub
[PubMed PMID: 33789010]
[73]
Liu A, Zhao J, Shah M, Migliorati JM, Tawfik SM, Bahal R, Rasmussen TP, Manautou JE, Zhong XB. Nedosiran, a Candidate siRNA Drug for the Treatment of Primary Hyperoxaluria: Design, Development, and Clinical Studies. ACS pharmacology & translational science. 2022 Nov 11:5(11):1007-1016. doi: 10.1021/acsptsci.2c00110. Epub 2022 Sep 21
[PubMed PMID: 36407951]
[74]
Shee K, Ahn J, Hamouche F, Mena J, Chi T, Stoller ML. Nedosiran Dramatically Reduces Serum Oxalate in Dialysis-Dependent Primary Hyperoxaluria 1: A Compassionate Use Case Report. Urology. 2021 Oct:156():e147-e149. doi: 10.1016/j.urology.2021.03.014. Epub 2021 Mar 25
[PubMed PMID: 33774044]
Level 3 (low-level) evidence
[75]
Baum MA, Langman C, Cochat P, Lieske JC, Moochhala SH, Hamamoto S, Satoh H, Mourani C, Ariceta G, Torres A, Wolley M, Belostotsky V, Forbes TA, Groothoff J, Hayes W, Tönshoff B, Takayama T, Rosskamp R, Russell K, Zhou J, Amrite A, Hoppe B, PHYOX2 study investigators. PHYOX2: a pivotal randomized study of nedosiran in primary hyperoxaluria type 1 or 2. Kidney international. 2023 Jan:103(1):207-217. doi: 10.1016/j.kint.2022.07.025. Epub 2022 Aug 22
[PubMed PMID: 36007597]
Level 1 (high-level) evidence
[76]
Bobrowski AE, Langman CB. Hyperoxaluria and systemic oxalosis: current therapy and future directions. Expert opinion on pharmacotherapy. 2006 Oct:7(14):1887-96
[PubMed PMID: 17020415]
Level 3 (low-level) evidence
[77]
Saborio P, Scheinman JI. Transplantation for primary hyperoxaluria in the United States. Kidney international. 1999 Sep:56(3):1094-100
[PubMed PMID: 10469379]
[78]
Garrelfs SF, Rumsby G, Peters-Sengers H, Erger F, Groothoff JW, Beck BB, Oosterveld MJS, Pelle A, Neuhaus T, Adams B, Cochat P, Salido E, Lipkin GW, Hoppe B, Hulton SA, OxalEurope Consortium. Patients with primary hyperoxaluria type 2 have significant morbidity and require careful follow-up. Kidney international. 2019 Dec:96(6):1389-1399. doi: 10.1016/j.kint.2019.08.018. Epub 2019 Sep 3
[PubMed PMID: 31685312]
[79]
Lieske JC, Goldfarb DS, De Simone C, Regnier C. Use of a probiotic to decrease enteric hyperoxaluria. Kidney international. 2005 Sep:68(3):1244-9
[PubMed PMID: 16105057]
[80]
Straub M, Hautmann RE, Hesse A, Rinnab L. [Calcium oxalate stones and hyperoxaluria. What is certain? What is new?]. Der Urologe. Ausg. A. 2005 Nov:44(11):1315-23
[PubMed PMID: 16235094]
[81]
Peck AB, Canales BK, Nguyen CQ. Oxalate-degrading microorganisms or oxalate-degrading enzymes: which is the future therapy for enzymatic dissolution of calcium-oxalate uroliths in recurrent stone disease? Urolithiasis. 2016 Feb:44(1):45-50. doi: 10.1007/s00240-015-0845-6. Epub 2015 Dec 8
[PubMed PMID: 26645869]
[82]
Whittamore JM, Hatch M. The role of intestinal oxalate transport in hyperoxaluria and the formation of kidney stones in animals and man. Urolithiasis. 2017 Feb:45(1):89-108. doi: 10.1007/s00240-016-0952-z. Epub 2016 Dec 2
[PubMed PMID: 27913853]
Level 3 (low-level) evidence
[83]
Burns Z, Knight J, Fargue S, Holmes R, Assimos D, Wood K. Future treatments for hyperoxaluria. Current opinion in urology. 2020 Mar:30(2):171-176. doi: 10.1097/MOU.0000000000000709. Epub
[PubMed PMID: 31895888]
Level 3 (low-level) evidence
[84]
Lindsjö M, Fellström B, Ljunghall S, Wikström B, Danielson BG. Treatment of enteric hyperoxaluria with calcium-containing organic marine hydrocolloid. Lancet (London, England). 1989 Sep 23:2(8665):701-4
[PubMed PMID: 2570957]
[85]
Letavernier E, Daudon M. Stiripentol identifies a therapeutic target to reduce oxaluria. Current opinion in nephrology and hypertension. 2020 Jul:29(4):394-399. doi: 10.1097/MNH.0000000000000621. Epub
[PubMed PMID: 32452916]
Level 3 (low-level) evidence
[86]
Poonguzhali PK, Chegu H. The influence of banana stem extract on urinary risk factors for stones in normal and hyperoxaluric rats. British journal of urology. 1994 Jul:74(1):23-5
[PubMed PMID: 8044524]
[87]
Malini MM, Baskar R, Varalakshmi P. Effect of lupeol, a pentacyclic triterpene, on urinary enzymes in hyperoxaluric rats. Japanese journal of medical science & biology. 1995 Oct-Dec:48(5-6):211-20
[PubMed PMID: 8718554]
Level 2 (mid-level) evidence
[88]
Ramakrishnan V, Lathika KM, D'Souza SJ, Singh BB, Raghavan KG. Investigation with chitosan-oxalate oxidase-catalase conjugate for degrading oxalate from hyperoxaluric rat chyme. Indian journal of biochemistry & biophysics. 1997 Aug:34(4):373-8
[PubMed PMID: 9491647]
[89]
Roodnat JI, de Mik-van Egmond AME, Visser WJ, Berger SP, van der Meijden WAG, Knauf F, van Agteren M, Betjes MGH, Hoorn EJ. A Successful Approach to Kidney Transplantation in Patients With Enteric (Secondary) Hyperoxaluria. Transplantation direct. 2017 Dec:3(12):e331. doi: 10.1097/TXD.0000000000000748. Epub 2017 Nov 8
[PubMed PMID: 29536032]
[90]
Andersson H, Jagenburg R. Fat-reduced diet in the treatment of hyperoxaluria in patients with ileopathy. Gut. 1974 May:15(5):360-6
[PubMed PMID: 18668844]
[91]
Morgan MS, Pearle MS. Medical management of renal stones. BMJ (Clinical research ed.). 2016 Mar 14:352():i52. doi: 10.1136/bmj.i52. Epub 2016 Mar 14
[PubMed PMID: 26977089]