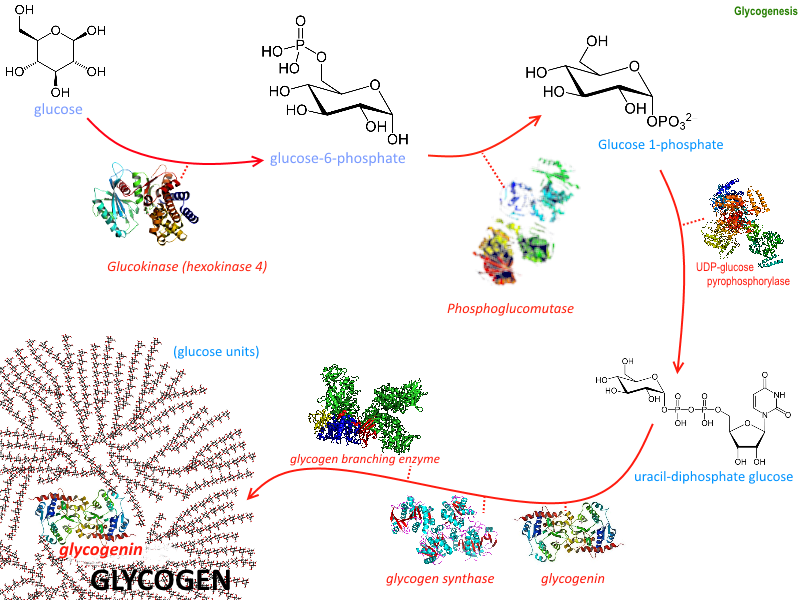
Introduction
Glycogen, also known as animal starch, is a branched polysaccharide that serves as a reserve of carbohydrates in the body; it is stored in the liver and muscle and readily available as an immediate energy source. The formation of glycogen from glucose is known as glycogenesis, and the breakdown of glycogen to form glucose is called glycogen metabolism or glycogenolysis. Increased cyclic adenosine monophosphate (cAMP) catalyzes the breakdown of glycogen (glycogenolysis). The primary hormones that regulate the cycle of glycogenesis and gluconeogenesis are insulin, glucagon, and cortisol. Glycogenolysis is initiated by the action of a specific enzyme called phosphorylase, which yields glucose-1-phosphate (P). Glucose-1-P is an essential compound at the intersection of several metabolic pathways, such as glycolysis, glycogenesis, glycogenolysis, and gluconeogenesis. When glycogenesis occurs, glycogenolysis is inhibited, and vice versa. Impaired glycogen metabolism, characterized by abnormal glycogen accumulation, is associated with inherited metabolic disorders and is collectively known as glycogen storage diseases.
Fundamentals
Glycogenolysis (glycogen metabolism) is initiated by the action of an enzyme known as phosphorylase. Phosphorylase catalyzes the phosphorolytic cleavage of α 1→ 4 linkage to produce glucose-1-P. The liver and muscle both contain the enzyme phosphorylase. Phosphorylase activity is regulated by phosphorylation; in the phosphorylated form, it is active, and in the non-phosphorylated form, it is inactive. The liver breaks down glycogen during periods of fasting to increase the blood glucose concentration for use as fuel by the body, particularly in the brain and red blood cells.
Muscles use glycogen to generate energy, particularly during anaerobic glycolysis in a "flight or fight" situation like intense exercise. Adrenaline triggers the breakdown of glycogen by binding to its receptor and activating adenylate cyclase, which produces cyclic adenosine monophosphate (cAMP). Increased cAMP activates a cascade of biochemical events that leads to stimulation of phosphorylase activity, which causes the breakdown of glycogen. Shortly after a meal high in carbohydrates, the liver may contain as much as 4% to 6% of glycogen. However, after approximately 12 to 18 hours of fasting, the liver is almost totally depleted of glycogen. Glycogenolysis, along with glycolysis, plays a central role in carbohydrate metabolism.[1]
Cellular Level
Glycogenolysis can occur in cytosol and lysosomes. However, different enzymes perform glycogen degradation in these cellular locations. Upon glycogen degradation, glucose or glucose-1-phosphate serves as fuel. In skeletal muscle, glucose uptake gets mediated via GLUT1 and GLUT4 transporters. In the cytosol, glycogen phosphorylase is activated by AMP for muscle tissue. For the liver, glucagon activates glycogen phosphorylase via increased cyclic-AMP (cAMP). Glycogen degradation in lysosomes is mediated by acid alpha-glucosidase through autophagic vacuoles that engulf cytoplasmic content and fuse with organelles.[2]
Molecular Level
Steps of Glycogenolysis
Phosphorylase kinase (PhK) is an enzyme that activates phosphorylase by phosphorylation. Various hormones and neuronal signals stimulate phK by producing the second messengers Ca2+ and cAMP. PhK consists of 4 subunits in a hexadecameric complex (αβγδ). Each PhK subunit has different isoforms or splice variants differentially expressed in human tissues.
Glycogenolysis occurs in the following steps:
- Activated glycogen phosphorylase breaks the glucosyl α-1→4 linkages and removes by phosphorolytic cleavage the 1→4 glucosyl residues from the outermost chains of the glycogen molecule until approximately 4 glucose residues remain on either side of an α-1→6 branch. It should be noted that phosphorylase activity produces glucose in the form of glucose-1-P, not as free glucose.
- When 4 glucose residues are left from the branch point, then another enzyme, α-1,4 →α-1,4 glucan transferase transfers a trisaccharide unit from one side to the other, exposing α-1→6 branch point.
- A specific debranching enzyme (amylo-1→6-glucosidase) hydrolyzes α-1→6 glucosidic linkage. This reaction produces 1 free glucose molecule.
- The combined action of phosphorylase and other enzymes convert glycogen to glucose-1-P. Phosphoglucomutase enzyme transfers a phosphate group on an α-D-glucose monomer from the 1 to the 6 position in the forward or opposite direction.
In the liver and kidney, glucose-6-phosphatase is present, which removes the phosphate group from glucose-6-P, forming free glucose. Free glucose diffuses to the extracellular space, marking the final step of hepatic glycogenolysis and increasing blood glucose levels.
In muscles, the enzyme glucose-6-phosphatase is absent. Therefore, glucose-6-P enters into the glycolytic cycle and forms pyruvate and lactic acid. But indirectly, lactic acid can serve as an intermediate molecule for glucose formation in the liver. Muscle glycogen cannot provide blood glucose by glycogenolysis due to a lack of the enzyme glucose-6-phosphatase.
It is important to note that besides phosphorolytic cleavage, 2 additional pathways for glycogenolysis have been discovered, involving α-amylase (α-amylolysis) and γ-amylase (γ-amylolysis).[3]
Function
Glycogen degradation occurs during fasting or when there is a low ratio of insulin to glucagon. The primary energy reserves for the body are glycogen and lipids. When lipids undergo oxidation, they produce a greater number of ATP molecules as compared to glycogenolysis, which produces a lower number of ATP molecules. However, 2 significant advantages exist for the metabolism of glycogen. The first advantage corresponds to the rapid mobilization of glycogen for metabolic requirements. Rapid mobilization is possible since glycogenolysis enzymes can adhere to the numerous branches of glycogen and begin simultaneous hydrolysis. The second advantage entails energy production under low lipid deposit conditions, such as anorexia.[3]
Glycogen levels are quantitively more present in skeletal muscle than in the liver. Nevertheless, glycogenolysis serves important roles in both tissues. In the liver, glycogen metabolism is vital during fasting conditions, leading to hepatic glucose production to maintain healthy blood glucose levels and support the body's energy needs. On the other hand, glycogen in skeletal muscle indicates a critical function in terms of rapid ATP generation. A close relationship exists between glycogen storage in skeletal muscle and fatigue resistance. The ability of a muscle to exercise during the first 30 minutes of activity, despite the abundance of other energy sources such as lipids, is severely compromised when glycogen levels are diminished in skeletal muscle. Glycogen depletion is shown to cause fatigue because the muscle cannot provide adequate fuel to skeletal muscle for excitation and contraction. A possible reason is related to the role of glycogen in releasing calcium from the sarcoplasmic reticulum.[4][5]
Aside from repleting energy, glycogenolysis leads to precursors for oxidative reactions of the pentose phosphate pathway, helping generate NADPH. These pathways are necessary for synthesizing fatty acids and producing pentose phosphates, essential for synthesizing RNA and DNA.[3]
Mechanism
The enzymes glycogen synthase and glycogen phosphorylase regulate the balance of glycogen metabolism. Glycogenesis responds to hormonal control and second messenger signals. One of the primary forms of control is the varied phosphorylation of glycogen synthase and glycogen phosphorylase. Enzymes under hormonal control regulate phosphorylation, and there are many different effectors compared to allosteric regulation systems.
Corresponding to cytosolic degradation, glycogen phosphorylase, the rate-limiting enzyme of glycogenolysis, cleaves terminal glucose residue connected to a glycogen branch while substituting a phosphoryl group for the α-1-4 bond. Four residues before an α-1-6 bond, corresponding to a branch, glycogen debranching enzyme catalyzes the transfer of 3 of the 4 remaining glucose residues to the end of another glycogen chain, which can be degraded by glycogen phosphorylase. In other words, the breakage of α-1-4 glycosidic bonds present in linear chains is catalyzed by glycogen phosphorylase, and the addition of the phosphate group to position 1 results in the production of glucose-1-phosphate.
The activity of glycogen phosphorylase is modulated allosterically and by phosphorylation. Glycogen production, conversely, inhibits glycogen degradation. Phosphoglucomutase is in charge of converting glucose-1-phosphate to glucose-6-phosphate through an isomerization reaction that has no energy requirements. On the other hand, the debranching enzyme deals with α-1-6 bonds and transfers a branch to the end of the polymer so that glycogen phosphorylase can continue working. In most tissues, glucose-6-phosphate is used for glycolysis and energy production. It is also a critical metabolic intermediate for other pathways, including the TCA cycle, fatty acid synthesis, Cori cycle, and alanine cycle.
Nevertheless, in gluconeogenic organs such as the liver, kidney, and intestines, glucose-6-phosphate is dephosphorylated to glucose—with the aid of the enzyme glucose-6-phosphatase—so that it can undergo transport from the endoplasmic reticulum to the interstitial space. Corresponding to the lysosomal glycogen degradation, the primary enzyme involved in acid maltase. The hydrolysis of glycogen to glucose, catalyzed by the acid alpha-glucosidase, is hypothesized to serve as a protective mechanism for the liver from high glycogen concentrations. Of the total glycogenolysis in skeletal muscle, only 5% of glycogen degradation happens in lysosomes. For liver glycogenolysis, only 10% occurs in lysosomes.[6][5][7]
Blood glucose level is maintained within physiological limits of 60 mg% to 100 mg% in fasting state and 100 mg% to 140 mg% following ingestion of a carbohydrate-containing meal by a balance between 2 sets of features: (A) rate of glucose entry into the bloodstream and (B) rate of glucose removal from the bloodstream.
Testing
Glycogen molecules cannot be visualized by light microscopy; electron microscopy is preferred. Histological staining and light microscopy only allow for the visualization of conglomerates of glycogen particles. Molecules of glycogen require electron microscopy. Depending on the tissue sample gathered, glycogen has been described as rosette-like beta particles or larger alpha particles. Rosette-like particles are found in muscle, while alpha particles, aggregates of beta particles, are found in the liver. Beta particles correspond to the typical configuration of glycogen with average chain lengths of 13 residues consisting of inner chains with branch points and outer chains without branch points. The designated stain for histological identification is Period Acid Schiff. However, certain disadvantages include a lack of specificity and general incompatibility with immunofluorescence techniques. A novel method for detecting glycogen in cells is now available. A renewable, recombinant protein-containing carbohydrate-binding module from starch-binding domain protein 1 (Stbd1) is employed to perform an enzyme-linked immunosorbent assay.[1][8]
Concerning testing for glycogen storage diseases, current DNA mutational analysis methods have eliminated the need to perform liver biopsies. This diagnostic test, for instance, applies to von Gierke disease and Cori disease. Diagnostic tests for Pompe disease include analysis of acid maltase activity in leukocytes or fibroblasts. A muscle biopsy showing excessive lysosomal glycogen accumulation is a diagnostic test for Pompe disease, specifically vacuolated myopathy.[5]
The rate of glycogenolysis is the difference between the rates of glucose production and absolute gluconeogenesis. Different techniques, such as radioactive and stable isotopes, are used to detect the levels of gluconeogenesis. Quantification of glycogenolysis is also possible via nuclear magnetic resonance spectroscopy.[9]
Pathophysiology
Impaired glycogenolysis can lead to a variety of diseases, including glycogen storage diseases (GSDs), lysosomal storage diseases, and Lafora progressive myoclonus epilepsy. Disruptions in glycogenolysis frequently affect the function of organs, including the liver, skeletal muscle, brain, and kidney. Depending on the affected enzyme in glycogenolysis, a spectrum of syndromes is possible.
von Gierke disease is the most common GSD. Type I GSD leads to a deficiency in glucose-6-phosphatase, which is responsible for dephosphorylating glucose-6-P so that glucose can get transported extracellularly to regulate blood glucose levels. The impaired ability to generate glucose results in severe hypoglycemia, hyperuricemia, and increased levels of lactic acid and triglycerides. Due to fat deposition, patients present with a rounded, doll-like face. Without treatment, failure to thrive, hepatomegaly, abnormal swelling, and delayed motor development are present. Long-term complications can develop due to kidney glycogen accumulation, leading to nephropathy, chronic kidney disease, and renal cancer. The main treatment involves maintaining normal glucose levels while avoiding hypoglycemia by instituting frequent meals.[5][10]
While glycogen degradation by phosphorylase and debranching enzyme can occur in the cytosol, glycogen is also degraded via a lysosomal pathway, leading to a lysosomal storage disease called Pompe disease (glycogen storage disease Type II). In Pompe disease, a mutation involving lysosomal alpha-glucosidase—also known as acid maltase—develops. As a result, glycogen accumulates in the lysosome and its vesicles, leading to fatal outcomes, including cardiomyopathy and muscular hypotonia. The mechanism of glycogen transportation to lysosomes is not fully understood, but it is believed to occur via macroautophagy. During this process, the cargo is engulfed within double-membrane vesicles known as autophagosomes that fuse with the lysosome.[1]
Glycogen storage disease Type III, Cori disease, results from a glycogen debranching enzyme deficiency. As a result, this disease manifests with an accumulation of abnormal glycogen since glycogenolysis halts when glycogen phosphorylase encounters a branching point. The glycogen is then considered abnormal because it reflects very short outer chains. Patients with Cori disease develop ketotic hypoglycemia and hepatomegaly. In rare cases, it can lead to liver cirrhosis and hepatocellular carcinoma.[5]
Glycogen storage disease Type V, McArdle disease, develops due to a skeletal muscle glycogen phosphorylase deficiency. Patients display exercise intolerance, muscle weakness, cramping, and pain. Creatine kinase levels are elevated, and myoglobinuria can also develop. A typical symptom of McArdle disease is second wind, where patients can resume exercise when resting briefly. Ingestion of sucrose before exercise can help alleviate symptoms since this becomes the energy source during exercise before resorting to glycogen stores.
When glycogen phosphorylase is deficient in the liver, a different disease develops—Type VI GSD. Type VI GSD, or Hers disease, exhibits normal creatine kinase and uric acid levels. Patients present with growth retardation and liver enlargement. Hyperlipidemia and ketotic hypoglycemia also are common.[5]
In Lafora progressive myoclonus epilepsy, increased phosphorylation of glycogen is present in several tissues, leading to toxicity and cell death in neurons. Symptoms include ataxia, seizures, myoclonus, and dementia. Abnormal phosphorylation levels in glycogen lead to longer chains and irregular branch points that render the polymer insoluble and degradation-resistant. As a result, patients with this condition have a conglomerate of inclusion bodies called Lafora bodies.[5]
Clinical Significance
Glycogenolysis plays a central role in regulating glucose levels in the blood. In muscle cells, glycogenolysis delivers an immediate source of glucose-6-P for glycolysis, which provides energy for muscle contraction. Impaired glycogenolysis is associated with various inherited metabolic disorders, collectively known as glycogen storage diseases.