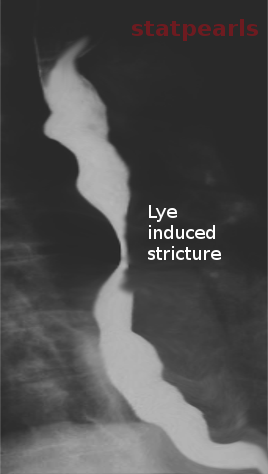
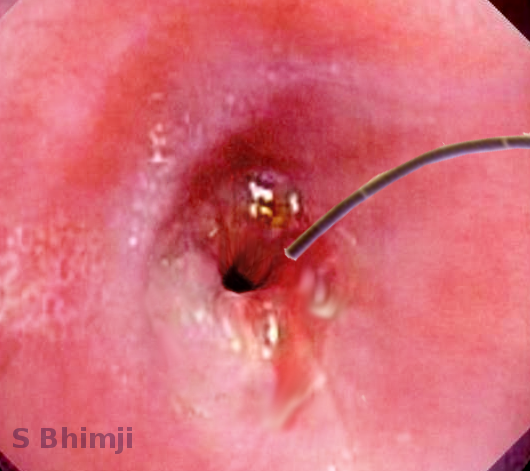
[1]
ASGE Standards of Practice Committee, Pasha SF, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Foley KQ, Fonkalsrud L, Hwang JH, Jue TL, Khashab MA, Lightdale JR, Muthusamy VR, Sharaf R, Saltzman JR, Shergill AK, Cash B. The role of endoscopy in the evaluation and management of dysphagia. Gastrointestinal endoscopy. 2014 Feb:79(2):191-201. doi: 10.1016/j.gie.2013.07.042. Epub 2013 Dec 12
[PubMed PMID: 24332405]
[2]
Lamoria S, De A, Agarwal S, Singh Lamba BM, Sharma V. Peptic esophageal stricture in an adolescent with Barrett's esophagus. International journal of adolescent medicine and health. 2016 Feb 27:29(5):. pii: /j/ijamh.2017.29.issue-5/ijamh-2015-0106/ijamh-2015-0106.xml. doi: 10.1515/ijamh-2015-0106. Epub 2016 Feb 27
[PubMed PMID: 26926861]
[3]
Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 31st Annual Report. Clinical toxicology (Philadelphia, Pa.). 2014 Dec:52(10):1032-283. doi: 10.3109/15563650.2014.987397. Epub
[PubMed PMID: 25559822]
[4]
Chirica M, Bonavina L, Kelly MD, Sarfati E, Cattan P. Caustic ingestion. Lancet (London, England). 2017 May 20:389(10083):2041-2052. doi: 10.1016/S0140-6736(16)30313-0. Epub 2016 Oct 26
[PubMed PMID: 28045663]
[5]
Le Naoures P, Hamy A, Lerolle N, Métivier E, Lermite E, Venara A. Risk factors for symptomatic esophageal stricture after caustic ingestion-a retrospective cohort study. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus. 2017 Jun 1:30(6):1-6. doi: 10.1093/dote/dox029. Epub
[PubMed PMID: 29207003]
Level 2 (mid-level) evidence
[6]
Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES, Dohil R, Falk GW, Gonsalves N, Gupta SK, Katzka DA, Lucendo AJ, Markowitz JE, Noel RJ, Odze RD, Putnam PE, Richter JE, Romero Y, Ruchelli E, Sampson HA, Schoepfer A, Shaheen NJ, Sicherer SH, Spechler S, Spergel JM, Straumann A, Wershil BK, Rothenberg ME, Aceves SS. Eosinophilic esophagitis: updated consensus recommendations for children and adults. The Journal of allergy and clinical immunology. 2011 Jul:128(1):3-20.e6; quiz 21-2. doi: 10.1016/j.jaci.2011.02.040. Epub 2011 Apr 7
[PubMed PMID: 21477849]
Level 3 (low-level) evidence
[7]
Lucendo AJ, Molina-Infante J, Arias Á, von Arnim U, Bredenoord AJ, Bussmann C, Amil Dias J, Bove M, González-Cervera J, Larsson H, Miehlke S, Papadopoulou A, Rodríguez-Sánchez J, Ravelli A, Ronkainen J, Santander C, Schoepfer AM, Storr MA, Terreehorst I, Straumann A, Attwood SE. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European gastroenterology journal. 2017 Apr:5(3):335-358. doi: 10.1177/2050640616689525. Epub 2017 Jan 23
[PubMed PMID: 28507746]
[8]
Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003 Dec:125(6):1660-9
[PubMed PMID: 14724818]
[9]
Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU, Straumann A. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec:145(6):1230-6.e1-2. doi: 10.1053/j.gastro.2013.08.015. Epub 2013 Aug 13
[PubMed PMID: 23954315]
[10]
Abid S, Mumtaz K, Jafri W, Hamid S, Abbas Z, Shah HA, Khan AH. Pill-induced esophageal injury: endoscopic features and clinical outcomes. Endoscopy. 2005 Aug:37(8):740-4
[PubMed PMID: 16032493]
Level 2 (mid-level) evidence
[11]
Kim SH, Jeong JB, Kim JW, Koh SJ, Kim BG, Lee KL, Chang MS, Im JP, Kang HW, Shin CM. Clinical and endoscopic characteristics of drug-induced esophagitis. World journal of gastroenterology. 2014 Aug 21:20(31):10994-9. doi: 10.3748/wjg.v20.i31.10994. Epub
[PubMed PMID: 25152603]
[12]
Choi GB, Shin JH, Song HY, Lee YS, Cho YK, Bae JI, Kim JH, Jeong YH, Park MH. Fluoroscopically guided balloon dilation for patients with esophageal stricture after radiation treatment. Journal of vascular and interventional radiology : JVIR. 2005 Dec:16(12):1705-10
[PubMed PMID: 16371539]
[13]
Park JH, Kim KY, Song HY, Cho YC, Kim PH, Tsauo J, Kim MT, Jun EJ, Jung HY, Kim SB, Kim JH. Radiation-induced esophageal strictures treated with fluoroscopic balloon dilation: clinical outcomes and factors influencing recurrence in 62 patients. Acta radiologica (Stockholm, Sweden : 1987). 2018 Mar:59(3):313-321. doi: 10.1177/0284185117713351. Epub 2017 Jun 2
[PubMed PMID: 28573925]
Level 2 (mid-level) evidence
[14]
Martínek J, Juhas S, Dolezel R, Walterová B, Juhasova J, Klima J, Rabekova Z, Vacková Z. Prevention of esophageal strictures after circumferential endoscopic submucosal dissection. Minerva chirurgica. 2018 Aug:73(4):394-409. doi: 10.23736/S0026-4733.18.07751-9. Epub 2018 May 24
[PubMed PMID: 29795068]
[15]
Yang D, Coman RM, Kahaleh M, Waxman I, Wang AY, Sethi A, Shah AR, Draganov PV. Endoscopic submucosal dissection for Barrett's early neoplasia: a multicenter study in the United States. Gastrointestinal endoscopy. 2017 Oct:86(4):600-607. doi: 10.1016/j.gie.2016.09.023. Epub 2016 Sep 28
[PubMed PMID: 27688205]
Level 2 (mid-level) evidence
[16]
Park JY, Song HY, Kim JH, Park JH, Na HK, Kim YH, Park SI. Benign anastomotic strictures after esophagectomy: long-term effectiveness of balloon dilation and factors affecting recurrence in 155 patients. AJR. American journal of roentgenology. 2012 May:198(5):1208-13. doi: 10.2214/AJR.11.7608. Epub
[PubMed PMID: 22528915]
[17]
Zieren HU, Müller JM, Pichlmaier H. Prospective randomized study of one- or two-layer anastomosis following oesophageal resection and cervical oesophagogastrostomy. The British journal of surgery. 1993 May:80(5):608-11
[PubMed PMID: 8518900]
Level 1 (high-level) evidence
[18]
Sedhom D, Sedhom R, Mishra A, Razjouyan H, Rustgi V. Esophageal Stricture Resulting from Systemic Chemotherapy for Solid Malignancy. ACG case reports journal. 2017:4():e99. doi: 10.14309/crj.2017.99. Epub 2017 Aug 16
[PubMed PMID: 28848771]
Level 3 (low-level) evidence
[19]
Kelly K, Storey L, O' Sullivan M, Butler K, McDermott M, Corbally M, McMahon C, Smith OP, O' Marcaigh A. Esophageal strictures during treatment for acute lymphoblastic leukemia. Journal of pediatric hematology/oncology. 2010 Mar:32(2):124-7. doi: 10.1097/MPH.0b013e3181ced25c. Epub
[PubMed PMID: 20168244]
[20]
Kitajima T, Momose K, Lee S, Haruta S, Shinohara H, Ueno M, Fujii T, Udagawa H. Benign esophageal stricture after thermal injury treated with esophagectomy and ileocolon interposition. World journal of gastroenterology. 2014 Jul 21:20(27):9205-9. doi: 10.3748/wjg.v20.i27.9205. Epub
[PubMed PMID: 25083096]
[21]
Moleiro J, Faias S, Afonso A. Esophageal Stricture of an Unusual Etiology. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016 Jun:14(6):e59-e60. doi: 10.1016/j.cgh.2015.11.006. Epub 2015 Nov 18
[PubMed PMID: 26598227]
[22]
Mbiine R, Kabuye R, Lekuya HM, Manyillirah W. Tuberculosis as a primary cause of oesophageal stricture: a case report. Journal of cardiothoracic surgery. 2018 Jun 5:13(1):58. doi: 10.1186/s13019-018-0743-4. Epub 2018 Jun 5
[PubMed PMID: 29871658]
Level 3 (low-level) evidence
[23]
Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018 Jan:154(2):267-276. doi: 10.1053/j.gastro.2017.07.045. Epub 2017 Aug 3
[PubMed PMID: 28780072]
[24]
Kuo WH, Kalloo AN. Reflux strictures of the esophagus. Gastrointestinal endoscopy clinics of North America. 1998 Apr:8(2):273-81
[PubMed PMID: 9583006]
[25]
Smith CD. Esophageal strictures and diverticula. The Surgical clinics of North America. 2015 Jun:95(3):669-81. doi: 10.1016/j.suc.2015.02.017. Epub 2015 Apr 15
[PubMed PMID: 25965138]
[26]
Gretarsdottir HM, Jonasson JG, Björnsson ES. Etiology and management of esophageal food impaction: a population based study. Scandinavian journal of gastroenterology. 2015 May:50(5):513-8. doi: 10.3109/00365521.2014.983159. Epub 2015 Feb 22
[PubMed PMID: 25704642]
[28]
Shami VM. Endoscopic management of esophageal strictures. Gastroenterology & hepatology. 2014 Jun:10(6):389-91
[PubMed PMID: 25013392]
[29]
Luedtke P, Levine MS, Rubesin SE, Weinstein DS, Laufer I. Radiologic diagnosis of benign esophageal strictures: a pattern approach. Radiographics : a review publication of the Radiological Society of North America, Inc. 2003 Jul-Aug:23(4):897-909
[PubMed PMID: 12853664]
[30]
Karasick S, Lev-Toaff AS. Esophageal strictures: findings on barium radiographs. AJR. American journal of roentgenology. 1995 Sep:165(3):561-5
[PubMed PMID: 7645471]
[31]
Sami SS, Haboubi HN, Ang Y, Boger P, Bhandari P, de Caestecker J, Griffiths H, Haidry R, Laasch HU, Patel P, Paterson S, Ragunath K, Watson P, Siersema PD, Attwood SE. UK guidelines on oesophageal dilatation in clinical practice. Gut. 2018 Jun:67(6):1000-1023. doi: 10.1136/gutjnl-2017-315414. Epub 2018 Feb 24
[PubMed PMID: 29478034]
[32]
Rana SS, Sharma R, Gupta R. High-frequency miniprobe endoscopic ultrasonography for evaluation of indeterminate esophageal strictures. Annals of gastroenterology. 2018 Nov-Dec:31(6):680-684. doi: 10.20524/aog.2018.0307. Epub 2018 Sep 14
[PubMed PMID: 30386117]
[33]
Standards of Practice Committee, Egan JV, Baron TH, Adler DG, Davila R, Faigel DO, Gan SL, Hirota WK, Leighton JA, Lichtenstein D, Qureshi WA, Rajan E, Shen B, Zuckerman MJ, VanGuilder T, Fanelli RD. Esophageal dilation. Gastrointestinal endoscopy. 2006 May:63(6):755-60
[PubMed PMID: 16650533]
[34]
Ogilvie AL, Dronfield MW, Ferguson R, Atkinson M. Palliative intubation of oesophagogastric neoplasms at fibreoptic endoscopy. Gut. 1982 Dec:23(12):1060-7
[PubMed PMID: 6184269]
[35]
Dakkak M, Hoare RC, Maslin SC, Bennett JR. Oesophagitis is as important as oesophageal stricture diameter in determining dysphagia. Gut. 1993 Feb:34(2):152-5
[PubMed PMID: 8432464]
[36]
Cox JG, Winter RK, Maslin SC, Dakkak M, Jones R, Buckton GK, Hoare RC, Dyet JF, Bennett JR. Balloon or bougie for dilatation of benign esophageal stricture? Digestive diseases and sciences. 1994 Apr:39(4):776-81
[PubMed PMID: 7818628]
[37]
Taub S, Rodan BA, Bean WJ, Koerner RS, Mullin DM, Feng TS. Balloon dilatation of esophageal strictures. The American journal of gastroenterology. 1986 Jan:81(1):14-8
[PubMed PMID: 3942119]
[39]
Tulman AB, Boyce HW Jr. Complications of esophageal dilation and guidelines for their prevention. Gastrointestinal endoscopy. 1981 Nov:27(4):229-34
[PubMed PMID: 7030864]
[40]
Spechler SJ. AGA technical review on treatment of patients with dysphagia caused by benign disorders of the distal esophagus. Gastroenterology. 1999 Jul:117(1):233-54
[PubMed PMID: 10381933]
[41]
Raymondi R, Pereira-Lima JC, Valves A, Morales GF, Marques D, Lopes CV, Marroni CA. Endoscopic dilation of benign esophageal strictures without fluoroscopy: experience of 2750 procedures. Hepato-gastroenterology. 2008 Jul-Aug:55(85):1342-8
[PubMed PMID: 18795685]
[42]
Pereira-Lima JC, Ramires RP, Zamin I Jr, Cassal AP, Marroni CA, Mattos AA. Endoscopic dilation of benign esophageal strictures: report on 1043 procedures. The American journal of gastroenterology. 1999 Jun:94(6):1497-501
[PubMed PMID: 10364013]
[43]
Yan X, Nie D, Zhang Y, Chang H, Huang Y. Effectiveness of an orally administered steroid gel at preventing restenosis after endoscopic balloon dilation of benign esophageal stricture. Medicine. 2019 Feb:98(8):e14565. doi: 10.1097/MD.0000000000014565. Epub
[PubMed PMID: 30813172]
[44]
Ramage JI Jr, Rumalla A, Baron TH, Pochron NL, Zinsmeister AR, Murray JA, Norton ID, Diehl N, Romero Y. A prospective, randomized, double-blind, placebo-controlled trial of endoscopic steroid injection therapy for recalcitrant esophageal peptic strictures. The American journal of gastroenterology. 2005 Nov:100(11):2419-25
[PubMed PMID: 16279894]
Level 1 (high-level) evidence
[45]
Tharavej C, Pungpapong SU, Chanswangphuvana P. Outcome of dilatation and predictors of failed dilatation in patients with acid-induced corrosive esophageal strictures. Surgical endoscopy. 2018 Feb:32(2):900-907. doi: 10.1007/s00464-017-5764-x. Epub 2017 Jul 21
[PubMed PMID: 28733733]
[46]
Kochman ML, McClave SA, Boyce HW. The refractory and the recurrent esophageal stricture: a definition. Gastrointestinal endoscopy. 2005 Sep:62(3):474-5
[PubMed PMID: 16111985]
[47]
Sharma P, Kozarek R, Practice Parameters Committee of American College of Gastroenterology. Role of esophageal stents in benign and malignant diseases. The American journal of gastroenterology. 2010 Feb:105(2):258-73; quiz 274. doi: 10.1038/ajg.2009.684. Epub 2009 Dec 22
[PubMed PMID: 20029413]
[48]
Hindy P, Hong J, Lam-Tsai Y, Gress F. A comprehensive review of esophageal stents. Gastroenterology & hepatology. 2012 Aug:8(8):526-34
[PubMed PMID: 23293566]
[49]
Repici A, Vleggaar FP, Hassan C, van Boeckel PG, Romeo F, Pagano N, Malesci A, Siersema PD. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointestinal endoscopy. 2010 Nov:72(5):927-34. doi: 10.1016/j.gie.2010.07.031. Epub
[PubMed PMID: 21034894]
[50]
Kantsevoy SV, Bitner M. Esophageal stent fixation with endoscopic suturing device (with video). Gastrointestinal endoscopy. 2012 Dec:76(6):1251-5. doi: 10.1016/j.gie.2012.08.003. Epub 2012 Sep 29
[PubMed PMID: 23031249]
[51]
Li X, Zhang W, Zhang G. Endoscopic suturing device with Overstitch for esophageal stent fixation. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2019 Jan:31(1):e3-e4. doi: 10.1111/den.13268. Epub 2018 Sep 30
[PubMed PMID: 30153347]
[52]
Imaz-Iglesia I, García-Pérez S, Nachtnebel A, Martín-Águeda B, Sánchez-Piedra C, Karadayi B, Demirbaş AR. Biodegradable stents for the treatment of refractory or recurrent benign esophageal stenosis. Expert review of medical devices. 2016 Jun:13(6):583-99. doi: 10.1080/17434440.2016.1184967. Epub 2016 May 18
[PubMed PMID: 27152556]
[53]
Karakan T, Utku OG, Dorukoz O, Sen I, Colak B, Erdal H, Karatay E, Tahtaci M, Cengiz M. Biodegradable stents for caustic esophageal strictures: a new therapeutic approach. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus. 2013 Apr:26(3):319-22. doi: 10.1111/j.1442-2050.2012.01418.x. Epub 2012 Sep 13
[PubMed PMID: 22974043]
[54]
Didden P, Reijm AN, Erler NS, Wolters LMM, Tang TJ, Ter Borg PCJ, Leeuwenburgh I, Bruno MJ, Spaander MCW. Fully vs. partially covered selfexpandable metal stent for palliation of malignant esophageal strictures: a randomized trial (the COPAC study). Endoscopy. 2018 Oct:50(10):961-971. doi: 10.1055/a-0620-8135. Epub 2018 Jun 12
[PubMed PMID: 29895072]
Level 1 (high-level) evidence
[55]
Pregun I, Hritz I, Tulassay Z, Herszényi L. Peptic esophageal stricture: medical treatment. Digestive diseases (Basel, Switzerland). 2009:27(1):31-7. doi: 10.1159/000210101. Epub 2009 May 8
[PubMed PMID: 19439958]
[56]
Frieling T. Non-Cardiac Chest Pain. Visceral medicine. 2018 Apr:34(2):92-96. doi: 10.1159/000486440. Epub 2018 Apr 12
[PubMed PMID: 29888236]
[57]
Swanström LL. Achalasia: treatment, current status and future advances. The Korean journal of internal medicine. 2019 Nov:34(6):1173-1180. doi: 10.3904/kjim.2018.439. Epub 2019 Mar 15
[PubMed PMID: 30866609]
Level 3 (low-level) evidence
[58]
Lawal A, Antonik S, Dua K, Massey BT. Esophageal adenocarcinoma: pseudo-nutcracker esophagus. Dysphagia. 2009 Jun:24(2):234-7. doi: 10.1007/s00455-008-9175-y. Epub 2008 Jul 15
[PubMed PMID: 18626696]
[59]
Wesdorp IC, Bartelsman JF, den Hartog Jager FC, Huibregtse K, Tytgat GN. Results of conservative treatment of benign esophageal strictures: a follow-up study in 100 patients. Gastroenterology. 1982 Mar:82(3):487-93
[PubMed PMID: 7054043]
[60]
Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointestinal endoscopy. 1998 Dec:48(6):563-7
[PubMed PMID: 9852444]
[61]
Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. The American journal of gastroenterology. 2013 Mar:108(3):308-28; quiz 329. doi: 10.1038/ajg.2012.444. Epub 2013 Feb 19
[PubMed PMID: 23419381]
[62]
Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA, American College of Gastroenterology. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). The American journal of gastroenterology. 2013 May:108(5):679-92; quiz 693. doi: 10.1038/ajg.2013.71. Epub 2013 Apr 9
[PubMed PMID: 23567357]