Introduction
Empyema is defined as a collection of pus in the pleural cavity, gram-positive, or culture from the pleural fluid. Empyema is usually associated with pneumonia but may also develop after thoracic surgery or thoracic trauma. In the United States, there are approximately 32,000 cases per year. Empyema is associated with elevated morbidity and mortality, around 20% to 30% of patients affected will either die or required further surgery in the first year after developing empyema. Early intervention is crucial in the management of empyema.[1][2]
Etiology
Around 20% of patients with pneumonia will develop a parapneumonic effusion that may lead to empyema. Seventy percent of patients with empyema have parapneumonic effusion, the other 30% of cases are related to trauma, post-thoracic surgery, esophageal ruptures, or cervical infections, and a small number are not related to previous pneumonia or intervention, this is known as primary empyema.[3][4]
Depending on if the infection is community-acquired or hospital-acquired, the bacteriology of empyema may change. Also, comorbidities of the patients need to be taken into consideration. For community-acquired empyema, gram-positive bacteria are more common, especially Streptococcus species. In this setting, the presence of gram-negative bacteria has been associated with increased comorbidities of patients with alcohol abuse, gastroesophageal reflux disease (GERD), and diabetes. In hospital-acquired empyema, Staphylococcus aureus, (methicillin-resistant S. aureus (MRSA)) and Pseudomonas are more common. When related to trauma and surgery S. aureus is also the most common agent.
The incidence of anaerobes when DNA amplification is implemented can be as high as 70%, when regular technics are used, the incidence can drop to 20%. Due to this discrepancy, it is always important to cover for anaerobe organisms despite negative cultures.
Fungal empyema is rare and is associated with high mortality, the most common fungus associated with it is Candida species.[5]
Epidemiology
Due to the association between pneumonia and empyema, patients at increased risk for pneumonia will also be at higher risk for empyema. Some risk factors have been identified as unique for developing empyema. Among these factors are diabetes mellitus, intravenous drug abuse, immunosuppression, gastric acid reflux, and alcohol abuse.
Pathophysiology
The development of empyema can be described in a sequence of events. During an inflammatory process such as pneumonia, there is an increase in fluid production in the pleural cavity known as the exudate stage. As the disease progresses microorganisms, usually bacteria, can colonize the fluid and generated an empyema. This fluid is characterized by elevated lactate dehydrogenase, proteins, neutrophils, and dead cells. Macroscopically is a thick opaque fluid found in the fibrinopurulent stage. After the resolution of the infection and as a consequence of the inflammation, there is a process of fibrosis that can lead to restriction of the lung parenchyma. Appropriate and early intervention is vital to decrease complications and mortality.[6][7]
History and Physical
The presentation may be similar to pneumonia, and cough, sputum production, fever, and pleuritic-type chest pain may be present. Patients with empyema may have symptoms for a more extended period. Research has shown that patients presented after a median of 15 days after the onset of symptoms. On physical exam there may be a dullness to percussion on the affected area, egophonia, increase palpable fremitus, and fine crackles. However, symptoms and physical exam findings are unspecific. Patients not improving with appropriate antibiotic therapy should be checked for the possibility of empyema.
A scoring system has been developed to assess mortality in 3 months upon presentation of patients with empyema. This system is called the RAPID score. The parameters included in the system are kidney function (Renal), age, presence or albescence of pus, hospital-acquired versus community-acquired infection, and albumin levels (diet). Patients with a score of more than 5 had poor outcomes.
Evaluation
Due to the lack of specificity in a clinical presentation, more tests are needed to establish a diagnosis.[8]
To evaluate for the presence of any pleural effusion, the first test that should be ordered is a chest x-ray. It is a widely available and simple test, but it is not 100% sensitive. A certain amount of fluid needs to be present to be detected, usually 75 ml in a lateral view, and approximately 175 ml in an anterior view. On an x-ray, some of the characteristics of a pleural effusion are blunted due to costodiaphragmatic angles and lungs filled with radiolucent fluid (depending on the size of the effusion).
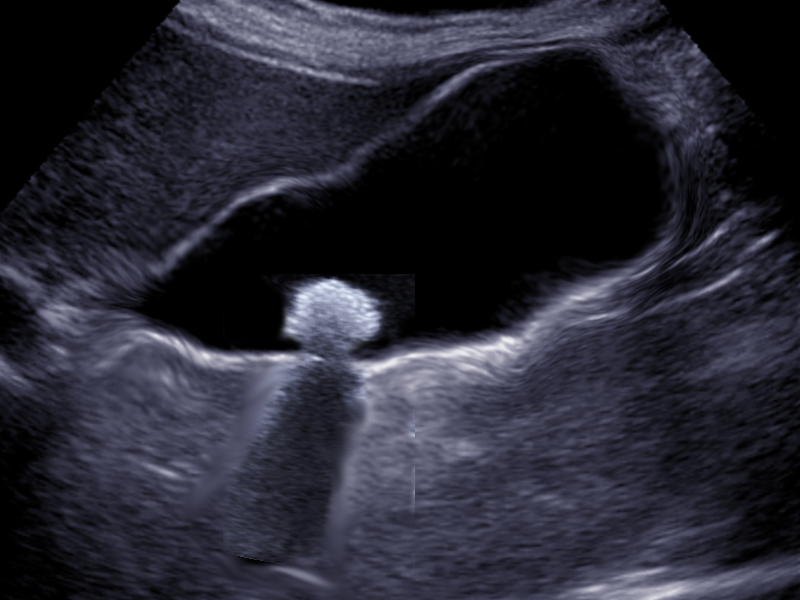
If an effusion is suspected with the chest x-ray, the next step is an ultrasound. Ultrasound is increasingly common because of its benefits, namely because it is widely available, it can be done at a patient's bedside, it is more sensitive at identifying pleural effusions than an x-ray, it allows differentiation between parenchyma and pleural fluid, and it also has a therapeutic use. Ultrasound can be useful in guiding a chest tube placement during thoracentesis. Some empyema characteristics found with ultrasound are homogenous echogenicity, anechoic effusion with hyperechoic septation, pleural thickening and split pleural, separation of the parietal, and visceral pleural by the fluid.
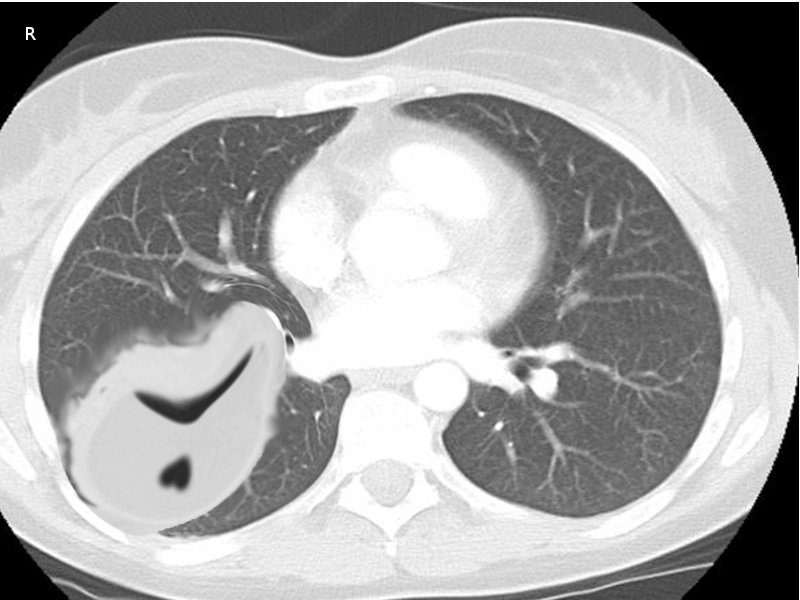
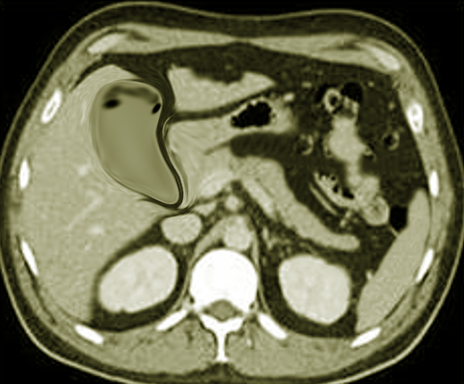
CT scan of the chest must be done in patients with empyema. It may be an alternative option after a chest -ray or ultrasound. CT scan ideally is done with intravenous (IV) contrast to enhance the pleura. CT scan can also be diagnostic and therapeutic, thoracentesis and tube thoracotomy can be performed under this modality. Some of the characteristics on CT scan are thickening of the pleura (present in approximately 80% to 100% patients), pleural enhancement, split pleural sign, bubbles in the absence of tube drainage, and septations. With a CT scan practitioners can better assess the lung parenchyma and the position of a chest tube.
After thoracentesis, the obtained fluid should be sent for analysis and culture.
Pleural fluid cultures have poor sensitivity; this can improve if the fluid is not the only stored in sterile containers but also in blood culture bottles. Ideally, the culture fluids should be obtained from the thoracentesis, chest tube placements, or surgical intervention, but never from pre-existing drainages.
Pleural fluid analysis is not necessary for the diagnosis of empyema, as mentioned before the presence of pus, gram-positive bacteria, or cultures will yield the diagnosis; nevertheless, all pleural fluids should be sent for analysis.
Treatment / Management
Like with all infections, prompt initiation of antibiotics and source control is fundamental. Treatment of empyema usually involves medical and surgical treatment. In community-acquired empyema, the use of a third or fourth-generation cephalosporin plus metronidazole or ampicillin with a beta-lactamase inhibitor will provided good coverage. In hospital-acquired or trauma-related, and surgery-related empyema, coverage of Pseudomonas and MRSA by adding vancomycin, cefepime, and metronidazole or piperacillin-tazobactam is essential . Due to the difficulty isolating anaerobes, the coverage for this organism should continue regardless of negative cultures. There is not a proven benefit of intrapleural antibiotics. Antibiotic should be given for 2 to 6 weeks, depending on patient response, source control, and organism. [9][10]
Tube thoracostomy is the most common type of drainage, bore tube versus smaller tubes have not shown any difference regarding mortality and prognosis, but bigger tubes are associated with more pain. For this reason, small tubes are frequently placed, generally smaller than 14F. The position of the tube should be confirming with an x-ray or CT scan. Lack of clinical improvement in the first 24 hours is usually related to tube malposition or blockage. Blockage of the chest tube can be prevented with frequent flushing, but the necessary amount and frequency of this process is unclear. Any indication of a persistent fluid or other locations should be addressed with more aggressive therapy including a larger tube, more tubes, or surgery. The chest tube can usually be removed when the daily production of pleural fluid is proximal 350 ml/day or less.
The use of intrapleural medication has been around for at least 60 years. Fibrinolytics such as urokinase, streptokinase, and tissue plasminogen activator have been tried, as well as, mucolytics such as DNase. The isolated use of fibrinolytics, so far, has not shown differences in mortality or surgical intervention. On the other hand, some studies have shown that the combination of fibrinolytic and mucolytic, specifically TPA and DNase increases the amount of fluid drainage and the need for surgery, unfortunately, no changes in mortality have been shown. Currently, the use of intrapleural agents is not a standard of care.
Usually, surgical intervention is the last resource. The main goal of surgical therapy in empyema is the evacuation of the pus from the pleural cavity and lung expansion. In patients requiring surgical intervention in acute empyema, video-assisted thoracotomy (VATS) is the first step. It is a less invasive procedure, there is less blood loss, less pain for the patient, better respiratory outcome, decrease length of stay, and a decreased 30 days mortality.
There is always a dilemma of when to convert to open-thoracotomy, and some of the clear indications for this are uncontrolled bleeding, damage to a structure that cannot be repaired with laparoscopy, and a patient who is not able to tolerate one-lung ventilation. Also, when the evacuation of the cavity or lung expansion are not achieved with VATS, an open-thoracotomy is indicated.
After the acute phase, some patients develop fibrosis and lung restriction that can cause dyspnea and exercise-intolerance as symptoms. Decortication may help to alleviate these symptoms and is considered when pulmonary restriction is present 6 months after the resolution of the infection but there are still issues in a patient's quality of life.
Differential Diagnosis
- Pneumonia
- Heart failure
- Pulmonary infarction
- Sequestration
Complications
- Fibrothorax
- Respiratory distress
Enhancing Healthcare Team Outcomes
Empyema is a common diagnosis in hospitals. Most empyemas follow bacterial pneumonia or unevacuated hemothorax following trauma. Because the condition can be debilitating, it is best managed by an interprofessional team. The key is early treatment before the onset of trapped lung. Thrombolytic therapy only works for early empyema but even when it works, a complete resolution is not always possible. A thoracic surgery consult should be made early; if the pleural cavity is emptied of all the fluid/blood, the risk of an empyema are low. However, if there is a delay in treatment, the patient may end up needing VATs or a thoracotomy. Patients with empyema usually have a chest tube and are monitored by nurses on the surgical floor. The prognosis for most patients with early empyema is excellent. However, complete resolution of the X-ray findings may take months. The pulmonary function usually improves rapidly once empyema is surgically managed.[1][11][12] (Level V)