Continuing Education Activity
Nasolacrimal duct obstruction (NLDO) often results in intractable, bothersome epiphora. In longstanding NLDO, mucus can accumulate, resulting in a mucocele in the nasolacrimal sac or even acute or chronic dacryocystitis. Dacryocystorhinostomy (DCR) surgery can be performed to restore tear drainage and is usually the definitive treatment. This activity highlights the importance of a collaborative interprofessional team approach to achieve safe and effective operation.
Objectives:
- Outline the historical developments leading to modern dacryocystorhinostomy.
- Describe the detailed clinical assessment of nasolacrimal duct obstruction.
- Explain the technical details of how to perform an external and endoscopic dacryocystorhinostomy.
- Review the importance of collaboration between the anesthetist and the surgeon when performing an external or endoscopic dacryocystorhinostomy.
Introduction
Dacryocystorhinostomy (DCR) describes the creation of a functional pathway from the canaliculi into the nose by means of creating an osteotomy and opening the nasolacrimal sac into the nose. It can be performed via an external or endonasal approach.
Obstruction of the excretory lacrimal system results in epiphora (tearing). Depending upon the exact cause and location of the obstruction, specific surgical procedures are used. These may include any of the following procedures:
- Punctoplasty
- Canalicular reconstruction
- Canaliculodacryocystorhinostomy
- External dacryocystorhinostomy
- Endoscopic dacryocystorhinostomy
- Conjunctivodacryocystorhinostomy
- Dacryocystectomy
This activity will address nasolacrimal duct obstruction (NLDO), which often results in intractable, bothersome epiphora. In longstanding NLDO, mucus can accumulate, resulting in a mucocele in the nasolacrimal sac or even acute or chronic dacryocystitis. Lacrimal surgery to restore tear drainage is usually the definitive treatment and involved one of the types of dacryocystorhinostomy.
History
The 12th-Century Andalusian oculist Muhamad Ibn Aslam Al Ghafiqi described the principles of lacrimal surgery in his book "The Right Guide to Ophthalmology." He reported using a small spear-shaped instrument perforating the lacrimal bone in a nasal direction "until blood flows through the nose and mouth with care given not to direct the instrument downward as this would be the incorrect direction." The probe was then wrapped in cotton that was either "dry or soaked in ox fat." This would then be exchanged every day to maintain the patency of the created fistula. This, then, could be described as the first description of creating an opening from the conjunctival fornix into the nose with secondary granulation and epithelialization, thereby forming a functioning fistula. Considering how little was known of the lining of the lacrimal passages and nose and just as little of the three-dimensional anatomy of the lacrimal system, this was a remarkable procedure. Indeed, this principle of fistulization remains the same to date as that of contemporary conjunctivodacryocystorhinostomy.[1][2][3]
The aim of performing a dacryocystorhinostomy is to create a fistula between the nasolacrimal sac and the nose, thus bypassing any obstruction and allowing passage of tears directly into the nose. The currently accepted technique of external-approach dacryocystorhinostomy (DCR) was first described at the beginning of the 20th century by the Florentine professor of otolaryngology, Addeo Toti in 1904 in the Italian literature, and later modified by Dupuy-Dutemps and Bourguet. Toti's procedure exposed the lacrimal sac via an external incision. He then excised the medial wall of the lacrimal sac and removed the adjoining lacrimal and maxillary bone, together with the mucosa: he achieved this with a hammer and chisel. The skin incision was then closed. Pressure was applied externally to push the lateral wall of the lacrimal sac inward, towards the nasal opening. The aim was to create pressure so that the lateral lacrimal sac became the lateral nasal wall with the direct opening of the canaliculi into the nose. The success was hampered by many factors, including the degree of bone and mucosal removal, secondary granulation formation, adhesions, and adequacy of external pressure. Various improvements in the original procedure were made:
- Toti modified the procedure in some cases with the removal of a portion of the middle turbinate and made wider bony windows.
- Kuhnt in 1914 introduced the suturing of the nasal mucosal flaps to the periosteum to reduce granulation tissue.
- Dupuy-Dutemps and Bourget modified Toti's operation in 1921 with vertical incisions in the nasal mucosa and the lacrimal sac together with horizontal incisions at the top and bottom ends of the vertical incisions, thereby creating "book-openings." They sutured both the anterior and posterior flaps of the nasal mucosa and the lacrimal sac. In 1933, they further modified their technique by incising the fistula created and probed the passage repeatedly via the lower punctum to reduce granulation and scar tissue and obtained a success rate of 95% in 1000 cases.
- Ohm, in 1962, essentially described a procedure very similar to the one described by Dupuy-Dutemps and Bourget and sutured the nasal mucosa to the lacrimal sac.
Endonasal DCR was first introduced by Caldwell in 1893, who used an endonasal electric burr to removed the bone once a metal probe had been passed through the canaliculus and into the lacrimal sac. Difficulties included adequate visualization, bleeding, accurate bone, and soft tissue removal. Although the technique was later modified by West in 1910 and Halle in 1914, real endonasal surgical improvements came with the rigid nasal endoscopes, which paved the way for advances in the field of endoscopic DCR. The modern-day approach to endonasal dacryocystorhinostomy was first reported by McDonogh and Meiring in 1989.[4] It now being accepted as an effective approach to DCR in the management of epiphora due to nasolacrimal duct obstruction.[5][6]
While in the 20th century, the most popular approach to DCR was that of an external technique, endonasal DCR, avoiding a skin incision has since been shown to be as successful as the external approach if an appropriate technique is used.[7][8] It should be emphasized that the term "endonasal" merely describes an approach through the nose rather than a specific technique. It is the evolution and expansion of the many endonasal techniques over time that has led to improved outcomes and greater acceptability and preference for an endonasal approach to DCR surgery.
Endonasal DCR was initially performed using rongeurs and was therefore termed "mechanical" endonasal DCR. With the advent of laser technology and the improvement of rigid nasal endoscopes, endoscopic "laser" DCR was popularised. However, lasers were unable to remove the thick bone of the frontal process of the maxilla and root of the middle turbinate, resulting in smaller bony ostea, extensive scarring, and, ultimately, higher failure rates.[9] This led to a shift to the principles of "powered" endoscopic DCR.[5] These principles mimicked those of external DCR, namely a large bony ostium, usually achieved by powered instrumentation, mucosal flaps, and mucosal edge-to-edge apposition, thereby aiming for primary intention healing and minimizing soft-tissue scarring. In an effort to reduce consumable costs, "powered" endoscopic DCR techniques have since evolved to use newer designed instruments, thus avoiding the need for powered instrumentation and, in effect, a shift back to mechanical endonasal DCR, while achieving full sac exposure and still creating mucosal flaps.[10]
Since the end of the 20th century, there has been a shift towards endoscopic DCR being accepted as being as safe and effective as external DCR. A large bony ostium (similar to that achieved in an external approach) is essential, and the mucosal flaps created intraoperatively should be well apposed for mucosal anastomosis. Endoscopic procedures that remove the adequate bone for full lacrimal sac exposure, marsupialization, and mucosal flap apposition have very high success rates, ranging between 90% to 100%.[11][12][13]
A recent study comparing various endoscopic DCR techniques reports equal safety and effectiveness.[14] Endoscopic DCR can also be effectively used in the pediatric population, in patients with craniofacial syndromes and syndromic nasolacrimal duct obstruction and the setting of distal canalicular or common canalicular obstruction.[15][16][17] While endoscopic DCRs are on the rise, the majority of DCR surgeries in the U.S.A. remain external.
Anatomy
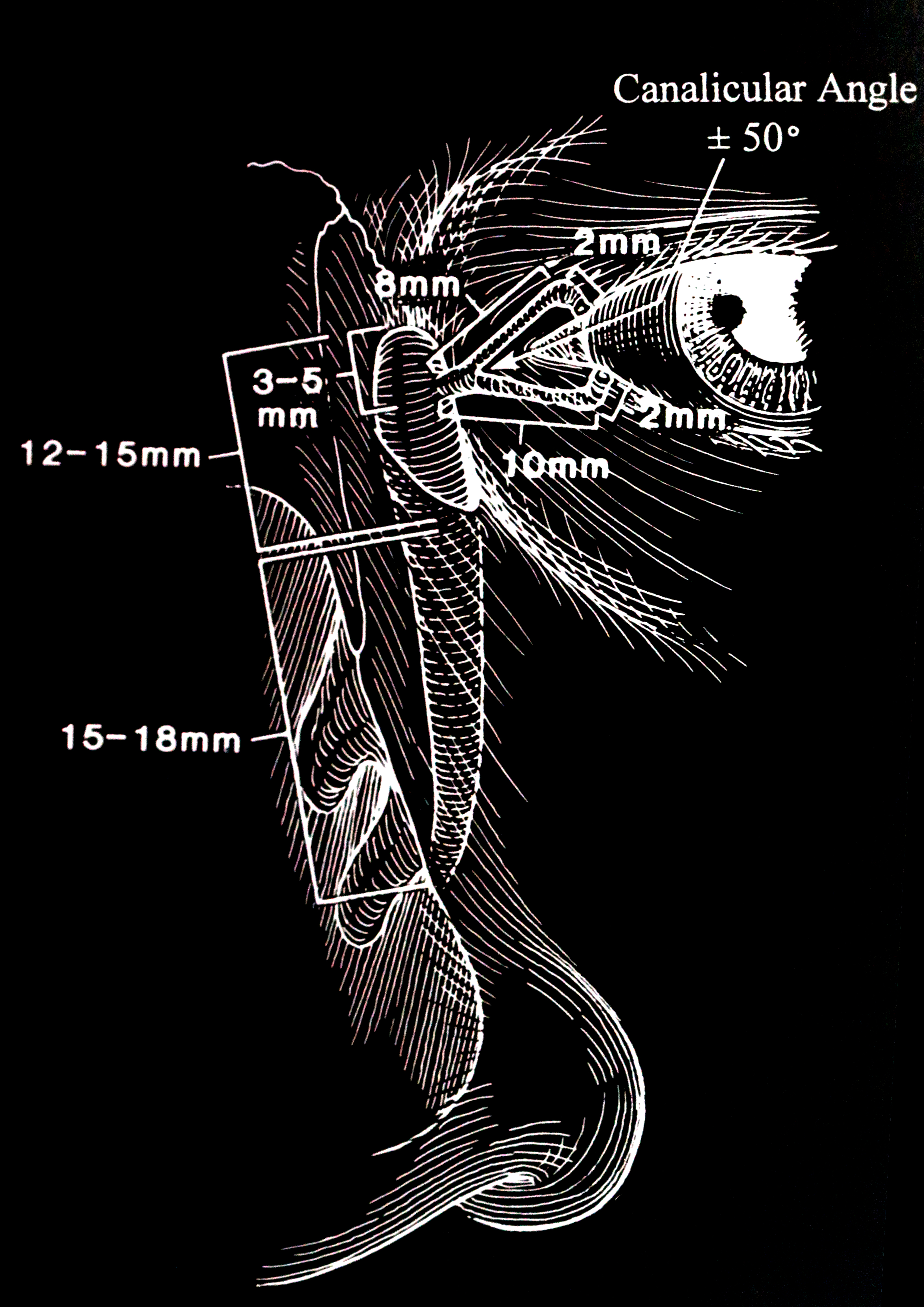
An appreciation of precise anatomy is essential to understand the process of DCR surgery. The lacrimal drainage pathway includes the lacrimal punctum (plural: puncti or puncta), the canaliculus and nasolacrimal sac, and finally, the nose. (fig 1)
Lacrimal Punctum
The punctum is located on the lacrimal papillae, facing slightly towards the globe, on both the upper and lower lids. This small aperture of approximately 0.3mm diameter allows the flow of tears into the canaliculus and is part of the lacrimal pump by means of a siphoning action.
Canaliculus
The upper and lower eyelids have one canaliculus each. These are lined with nonkeratinized squamous epithelium. The canaliculus initially travels about 2 mm vertically, and then turns horizontally in parallel with the eyelid margin. This horizontal component of the canaliculus is surrounded by Horner's muscle, which is part of the lacrimal part of orbicularis oculi. Horner's muscle contributes to the lacrimal pump function, and any dysfunction of orbicularis oculi may contribute to epiphora due to pump failure. In most individuals (94%), the canaliculus from the upper and lower lid converge and join to form the common canaliculus. The length of the upper canaliculus is about 8mm, whereas the lower canaliculus is about 10 mm.
The upper and lower canaliculi each angle slightly posteriorly, but the common canaliculus, in turn, may angle anteriorly. Awareness of this change in direction is essential for safe, atraumatic syringing and probing, which constitutes part of the assessment and pre-operative workup. The common canaliculus then pierces the periorbita and enters the lacrimal sac. However, in some individuals, separate upper and lower canaliculi may enter the sac. The entry into the lacrimal sac most often occurs obliquely, which forms the valve of Rosenmueller. While this is not a valve per se, the angulation of the common canaliculus as it enters the sac prevents retrograde flux and acts in a valve-like manner.
Lacrimal Sac and Duct
The nasolacrimal sac and duct are continuous rather than separate structures that are lined with non-ciliated columnar epithelium. The sac sits within the lacrimal fossa, which is formed by the lacrimal bone and the frontal process of the maxilla. Its dimensions are 12 to 15 mm in height and 4 to 8 mm anteroposteriorly. The superior fundus of the sac extends 3 to 5 mm above the medial canthal tendon. The nasolacrimal duct extends inferolaterally and posteriorly through the bone for approximately 12 mm, exiting underneath the inferior turbinate. This nasolacrimal duct ostium is located 25 to 30 mm posterior to the anterior nares. The exit of the nasolacrimal duct into the nose is can be round or slit-like and is protected by a mucous membrane covering, called the valve of Hasner or plica lacrimalis.
Endoscopic Nasal Anatomy
Endonasal anatomy is complex: a detailed discussion is beyond the scope of this chapter. However, a few salient points must be highlighted to understand DCR surgery. The review by Shams et al. is an excellent review of applied endonasal anatomy.[18]
The lacrimal fossa is formed by the frontal process of the maxilla anteriorly and the lacrimal bone posteriorly. The fossa is bordered by the anterior lacrimal crest and posterior lacrimal crest. The lacrimal fossa measures about 16 mm vertically is 2 to 4 mm deep with a width of 7 to 10 mm. Variability exists depending upon ethnic origins.[18]
The nose has three turbinates, each of which has a corresponding meatus inferior to it. The nasolacrimal duct opening lies within the inferior meatus, but it is the middle turbinate which may guide the DCR surgeon. The middle meatus contains the uncinate process, the bulla ethmoidalis, the frontal recess, and the maxillary sinus ostium. The axilla of the middle turbinate marks the point of the middle turbinate inserting into the frontal process of the maxilla and tends to be a constant anatomical landmark. The lacrimal fossa is situated superiorly, anteriorly and laterally to the axilla of the middle turbinate.
It is essential to appreciate the extent of the sac with respect to the middle turbinate to perform successful endoscopic DCR. The sac extends 8 to 10 mm above the superior extent of the middle turbinate and about 4 mm below its inferior border. This means that a significant part of the lacrimal sac fundus lies above the axilla of the middle turbinate, and it is essential to adequately expose this during DCR surgery in order to minimize failure.
The maxillary line is the most medial part of the frontal process of the maxilla, and it runs from the axilla of the middle turbinate along the lateral nasal wall ending at the inferior turbinate. It is an important landmark for the placement of mucosal incisions.
The uncinate process attaches to the lateral nasal wall at the frontal process of the maxilla. Inferiorly, it attaches to the ethmoidal process of the inferior turbinate. The infundibulum is an area lateral to the uncinate process, into which the maxillary sinus and anterior ethmoid air cells drain. During the creation of the bony ostium as part of endoscopic DCR surgery, the uncinate process helps define the posterior-inferior extent of bone removal involving the lacrimal bone covering the lacrimal sac. Once the uncinate process is encountered, further ostium creation can extend upwards.
The agger nasi cells are situated in the lateral nasal wall and are pneumatized to varying extents in different individuals. They may displace the insertion of the middle turbinate medially and can be seen well on computerized tomography (CT), anterior to the middle turbinate. They are usually closely situated near the posterior-superior aspect of the lacrimal fossa and may extend superiorly above the lacrimal fossa.
Etiology
The etiology of nasolacrimal duct obstruction is as follows:
- Congenital nasolacrimal duct obstruction (CNLDO): usually caused by a mucosal membrane at the inferior meatus but can also be caused by bony constriction of the nasolacrimal duct
- Primary acquired nasolacrimal duct obstruction (PANDO): usually caused by age-related fibrosis of the nasolacrimal duct
- Secondary acquired nasolacrimal duct obstruction (SANDO):
- Infection:
- Bacterial (Staphylococcus, Streptococcus, Actinomyces are the most frequent causes)
- Fungal
- Viral
- Parasitic
- Inflammation:
- Sarcoidosis
- Wegener granulomatosis (granulomatosis with polyangiitis - GPA)
- Allergies
- Ocular drops (glaucoma medication, chemotherapy drugs, antiviral drugs)
- Irradiation (use of I-131 for thyroid carcinoma)
- Physical burns
- Chemical burns
- Pemphigoid disease
- Stevens-Johnson disease
- Chemotherapeutic drugs (docetaxel for breast carcinoma and lung cancer)
- Neoplasia
- Lacrimal sac tumors (lymphoma, papilloma, squamous cell carcinoma, melanoma)
- Surrounding soft tissue tumors such as basal cell carcinoma, adenoid cystic carcinoma, lymphoma, leukemia
- Metastatic tumors (breast carcinoma, malignant melanoma, prostate carcinoma)
- Trauma
- Midfacial fractures including naso-orbito-ethmoid fractures
- Iatrogenic trauma
- Mechanical
- Surrounding mucoceles
- Dacryoliths within the lacrimal system
Epidemiology
The incidence of both acquired and congenital nasolacrimal duct obstruction is common, with slight female preponderance for the acquired type.
- For acquired nasolacrimal duct obstruction, the estimated incidence is 20.24 per 100 000 persons
- For congenital nasolacrimal duct obstruction, 5% to 20% (mean of 6%) of newborns are affected, but the majority of cases are self-resolving by the age of 12 months
- in 31.8% of cases of all chronic epiphora, the cause is nasolacrimal duct obstruction
- Nasolacrimal duct obstruction is the cause of tearing in all lacrimal passage obstructions
- Dacryocystitis occurs in 1/3884 of live births
- Acquired nasolacrimal duct obstruction more commonly affects Whites than African Americans
- There is no evidence that eye make-up causes nasolacrimal duct obstruction
Pathophysiology
Primary acquired nasolacrimal duct obstruction is caused by chronic low-grade inflammation and secondary fibrosis with no specific primary cause, as shown by Linberg and McCormick in 1986 on histopathology studies.[19] Primary acquired nasolacrimal duct obstruction occurs more in females. Groessl et al. showed that the lower nasolacrimal fossa and the middle section of the nasolacrimal duct are significantly smaller in women.[20] It is also thought that hormonal changes in women can lead to de-epithelialization of the lacrimal sac and lacrimal duct, thereby causing nasolacrimal duct obstruction, especially with an already narrower nasolacrimal canal.
History and Physical
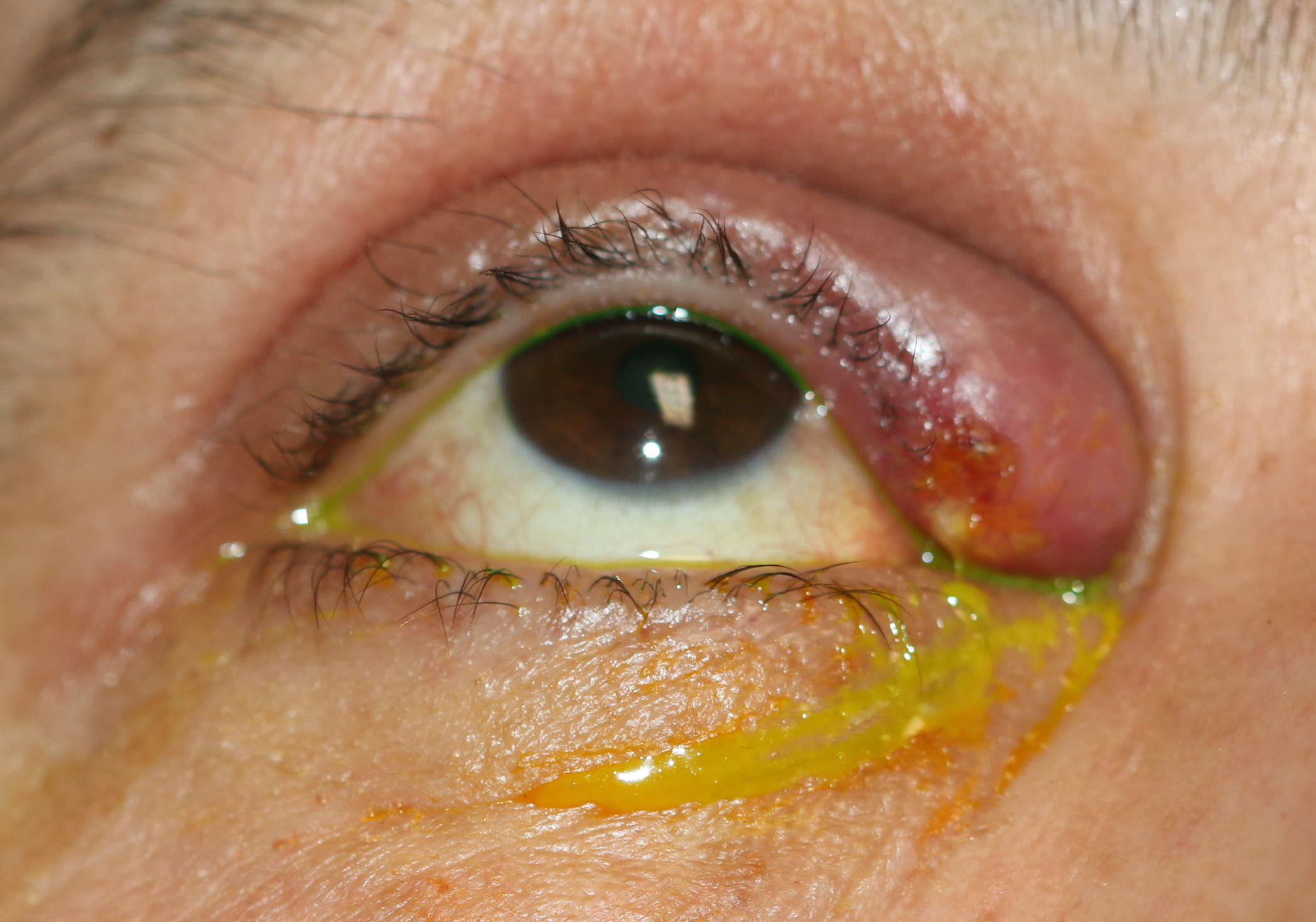
Nasolacrimal duct obstruction causes epiphora. Epiphora can be caused by problems with the secretory part of the lacrimal system or the excretory part. A combination of a detailed history and examination, together with appropriate investigations, will allow the surgeon to determine the cause of the epiphora. A detailed discussion of the history, physical findings, and relevant investigations is presented in the articles "Epiphora" and "Epiphora - clinical testing." Nasolacrimal duct obstruction may present with epiphora together with a mucoid discharge, and there may be a mucocele if there is a collection of mucous within the lacrimal sac. In the presence of dacryocystitis, there may be pain in the medial canthal region.
A detailed history is taken, and an ophthalmic and endoscopic nasal examination performed preoperatively.
Several pertinent points must be elicited as the clinical history of epiphora can often be diagnostic.
- Epiphora characteristics
- Laterality
- Duration
- Variability through the day
- Variability indoors vs. outside (often patients have a component of reflex tearing)
- Location (epiphora related to NLDO will result in overflow of tears medially or centrally over the lower lid, whereas lateral epiphora is more likely related to the "wick syndrome")
- Effects (previous dacryocystitis, repeated antibiotic prescriptions, repeated health care presentations, excoriation of the skin)
- In the pediatric patient: has the epiphora been present since birth? If so, has it improved?
- Past medical history
- Previous nose or sinus surgery
- Previous facial trauma or fractures
- Previous rhinitis/rhinosinusitis
- General health
- Current medications, in particular, anticoagulants
- In the pediatric patient: obstetric and birth history, prematurity, diagnosis of other medical issues, or syndromes.
- Previous treatments for epiphora
- Previous ophthalmic history particularly dry eyes, Meibomian gland dysfunction, previous refractive surgery
- Palpation for swelling of the lacrimal sac, mucocele, site of swelling (above or below the medial canthal tendon)
Evaluation
Tests for Nasolacrimal Duct Obstruction
Jones 1 and Jones 2 tests are used to determine if there is blockage of the nasolacrimal system as has been described elsewhere. The Jones 2 test with irrigation of the lacrimal system is especially helpful in determining the site of the obstruction. On rare occasions, a dacryocystogram may be needed, especially in the presence of fistulas, iatrogenic trauma, or suspected lacrimal sac tumors.
Preoperative Clinical Examination
- Alternative causes for epiphora, such as evaporative dry eye, reflex tearing, eyelid malposition, and punctal stenosis, must be excluded.
- The eyelids should be examined for malposition and evidence of Meibomian gland dysfunction.
- The punctum is inspected for size and location, specifically as to whether it is in the tear lake.
- Examine for the absence of puncta and any fistulae or accessory puncta, especially so in all children
- The cornea should be examined for punctate epithelial staining, tear film quality, tear break up time, epithelial defects, and infiltrate/keratitis.
- The fluorescein dye disappearance test should be evaluated. This is a physiological test of tear flow.
- The nasolacrimal sac area should be inspected for the presence of swelling or a mucocele. Any swelling above the medial canthal tendon is atypical and may represent malignancy of the sac mucosa or other pathology. This should be investigated with imaging prior to proceeding to DCR.
- Digital pressure is applied to the sac area, and the punctum is observed for any mucoid reflux indicative of a mucocele.
Diagnostic Irrigation of the Lacrimal System
A diagnostic syringing of the nasolacrimal system consists of the following steps:
- Anesthetize the conjunctiva with oxybuprocaine or tetracaine.
- If the punctum is stenosed or very small, gentle dilation with a punctal dilator may be required.
- Using a lacrimal cannula on a saline-filled syringe (2 ml or 3 ml), place the cannula through the lower punctum and into the mid canalicular area. Syringe and observe for:
- Passage of saline into the nose (should be graded as a percentage)
- Reflux via the lower punctum
- Reflux via the upper punctum
- Quality of reflux, e.g., mucoid or clear
- The ease of syringing.
- The pressure needed to irrigate: this is known as the "educated finger" that the examiner acquires over time.
- Advance the cannula into the sac. Determine whether the cannula truly is in the sac by feeling for a hard stop. A soft stop is indicative of a distal canalicular obstruction or, more commonly, a normal kinking of the distal common canaliculus, preventing the cannula passing into the sac.
- Once the cannula is within the sac, withdraw slightly to avoid abutting the wall of the sac and syringe again. Observe the same features as described in step 3.
- If there truly is a soft stop, syringing should be repeated via the upper punctum.
- Repeat on the opposite side.
Diagnostic Nasoendoscopy
Nasoendoscopy must be included in the preoperative workup. This is essential when an endonasal DCR technique is being considered, but also important even if an external DCR is planned. A decongestant plus anesthetic spray (oxymetazoline and lidocaine) gives the best decongestion and anesthesia in nasal endoscopy.[21]
A 30 degree, 2.7 mm slim nasoendoscope is introduced into the nose.
The anterior nose is observed for polyps or other abnormalities. The endoscope is then advanced, and the following observations are made:
- Is the septum straight, or does it deviate? Deviation towards the operative side may make access difficult and require a septoplasty.
- Is the mucosa healthy?
- Can the middle turbinate be identified?
- Is there sufficient space to perform endoscopic DCR?
- Are there any other abnormalities that may hinder surgery?
The Pediatric Patient
In the pediatric patient, most of the above investigations cannot be performed while the patient is awake. A thorough history from the child's caregiver is often diagnostic. The eyelids and periocular area should be inspected for mattering on the lashes and signs of excoriation secondary due to epiphora. The level of the tear film is observed and compared to the other side: a high tear film is indicative of NLDO. Sometimes a fluorescein dye disappearance test can be performed. For this, one drop of fluorescein is instilled into the eye, and its disappearance from the ocular surface is observed over the following minutes. It should always be compared to the contralateral eye.
All findings should be documented, and the patient counseled appropriately regarding their surgery. Informed consent must be obtained.
Evaluating the Patient for External vs. Endoscopic DCR
To effectively and permanently cure troublesome epiphora, a large fistula between the lacrimal sac and the nose is required. This can be achieved via the external and endonasal approach, with high success rates. The time taken for a DCR procedure varies with the experience of the surgeon. While less experienced surgeons take longer to perform an endoscopic DCR (95.7 ± 27.3 minutes), compared to an external approach, more experienced surgeons have comparable operating times (35 minutes).[22] Endoscopic DCR is favored for most clinical situations, as the rate of success is as high as external DCR but with faster rehabilitation and fewer postoperative follow-up visits required.[23][5] In lower resource settings, however, it is important to emphasize that external DCR can achieve comparable outcomes at a lower cost.[24]
Considerations for an External DCR
- In the elderly patient, unfit for general anesthesia, an external DCR is an ideal option as it can be performed with minimal sedation under local anesthesia. Any intraoperative bleeding can be controlled easily. While some centers perform endoscopic DCR under sedation, we do not advocate this option, as sedation may dampen the gag reflex, increasing the risk for pulmonary aspiration.
- Biopsy of the lacrimal sac, when needed, is perhaps somewhat easier with the external approach. Furthermore, biopsies can be performed prior to breaching bone, thus reducing the risk of spreading potential malignancy.
- In patients with previous facial fractures or unusual anatomy, the external approach may make surgery easier and more predictable.
- Where a septoplasty is required for adequate access to perform successful endonasal-approach DCR, an external approach may be preferred to avoid the need for a septoplasty.[25]
- In patients with proximal or mid canalicular stenosis, external DCR allows for retrograde intubation.
Considerations for an Endoscopic DCR
Endoscopic DCR is favored for most clinical situations, as the rate of success is as high as external DCR.[25] External DCR can cause orbicularis and, therefore, lacrimal pump dysfunction, which may further worsen epiphora.[5] Biopsies of the sac mucosa can be performed via both techniques if adequate equipment is available. For surgeons performing DCR on an only intermittent basis, biopsies taken via the external approach may be more reliable.
Several indications for endoscopic DCR include:
- Primary acquired nasolacrimal duct obstruction.
- Secondary acquired nasolacrimal duct obstruction
- Persistent congenital nasolacrimal duct obstruction
- Functional nasolacrimal duct obstruction
- Acute dacryocystitis, unresponsive to medical treatment
- Chronic dacryocystitis
- In the setting of acute dacryocystitis, endoscopic DCR is particularly useful, as it drains an anaerobic abscess cavity into an aerated space, creating excellent drainage without involving other tissue planes and giving long term relief from epiphora.[26]
Contraindications
- Other causes for epiphora, such as evaporative dry eye, must be excluded before DCR surgery.
- In the setting of previous cutaneous malignancy of the medial canthus, DCR surgery should only be carefully considered after follow-up confirms no suspicion of recurrence.
- Breaching the bony barrier (and skin in the setting of external DCR) may potentially spread tumors in the setting of recurrent disease.
- Prior radiotherapy in the medial canthal region may reduce vascularity and healing in this area.
Surgical Technique
Equipment for External DCR
- Moffett's solution / Cocaine drops or paste (not essential but useful)
- Chlorhexidine skin preparation solution
- Turban head drape
- 15 blade
- Cotton buds
- Gauze
- Rollett's rongine
- Kerrison bone punch
- Lacrimal probes
- Punctum dilator
- St Martin forceps
- Adson forceps
- Needle holder
- Wescott scissors
- 6-0 polyglactin 910 suture on half circle needle
- 6-0 polypropylene suture
Endoscopic DCR
- Moffett's solution or cocaine drops or paste
- Chlorhexidine skin preparation solution
- Turban head drape
- An endoscope (30 degrees)
- Light lead
- Light source stack
- Endoscrub machine
- St. Martin forceps
- Malhotra bone nibbler
- Kerrison bone punch
- Neurosurgical patties
- Crescent knife
- 15 blade on a long handle
- Suction freer
- Blakesley forceps
- Lacrimal probes
- Punctum dilator
- Long dental needle
- Dental syringe
- Mini-Monoka or Crawford tubes for nasolacrimal intubation
- Gelsponge soaked with triamcinolone
Anesthesia
General Considerations
Bleeding, when operating in a confined space such as during endoscopic or even external DCR, can obscure the surgical view and make a procedure much more difficult. Surgical bleeding can be categorized into arterial, venous, and capillary bleeding. Especially when performing surgery under general anesthesia, the anesthetist is essential. The anesthetist may induce controlled hypotension to reduce intraoperative bleeding; This is defined as a reduction of the systolic blood pressure to 80 to 90mmHg or a 30% reduction baseline mean arterial pressure. As to what is safe, controlled hypotension, much discussion exists and depends on the patient's general health and cardiovascular status. Especially for venous and capillary bleeding, heart rate is an important variable. When performing endoscopic DCR in children, it is essential to recall that children require their faster heart rate to maintain perfusion and cardiac output. Therefore, heart rate should not be reduced in children and may, in turn, lead to more intraoperative bleeding.
Both external and endoscopic DCR can be performed on anticoagulated patients, but the cessation of anticoagulation must be individualized depending on patient risk factors.[27][28] It is strongly recommended to liaise with the patient's prescribing physician or cardiologist prior to interrupting anticoagulation in patients with previous cardiac or stroke history.
All non-essential supplements that may contribute to bleeding including garlic, ginkgo bilbo, turmeric, ginger, ginseng, fish oil and krill oil, evening primrose, and St John's wort should be stopped several weeks pre-operatively.[28]
Selective serotonin reuptake inhibitors (SSRIs) are known to increase the risk of perioperative hemorrhage.[29] SSRIs (e.g., citalopram, fluoxetine, fluvoxamine, and sertraline) are commonly used to treat mood disorders such as depression, anxiety, and obsessive-compulsive disorders. It has been postulated that SSRIs cause depletion of platelet serotonin, leading to a reduced ability to form clots and a subsequent increase in the risk of bleeding. Serotonin release normally helps induce vasoconstriction and platelet activation, and to enhance fibrin formation. This important neurotransmitter also helps in generating coated platelets, a subgroup of platelets with important procoagulant activity. Although some clinical practice references suggest withholding SSRIs for 2 or more weeks before surgery, it is difficult to frame a detailed strategy based on the available evidence.[30] Discontinuing SSRIs could lead to discontinuation syndrome, increased sensitivity to pain, and relapsing depression postoperatively. Consultation with a psychiatrist or the prescribing physician is recommended prior to DCR surgery.
Perioperative Considerations
The patient should be placed in a reverse-Trendelenburg position of approximately 10 to 15 degrees to increase venous return to the heart and reduce intraoperative bleeding.[31] The use of a laryngeal mask airway may induce less sympathetic stimulation during placement compared to an endotracheal tube, which in turn appears to translate to lower heart rate and less bleeding during the initial 15 minutes of the procedure. Furthermore, it may protect the upper airway from blood coming from above during endoscopic DCR surgery. However, the anesthetist and surgeon must be aware that during the endoscopic DCR procedure, in particular, the head will be moved side to side due to pressure applied during bony ostium creation. This factor, along with the fact that positive pressure ventilation can be applied, may encourage some to prefer an endotracheal tube. Total intravenous anesthesia improves the surgical field (as inhalation agents have a vasodilatory effect) and reduces postoperative nausea and vomiting.
Adjunct medications such as tranexamic acid have a beneficial effect on intraoperative and postoperative bleeding. The authors give tranexamic acid routinely during endoscopic DCR surgery.
Postoperatively, most patients are comfortable if appropriate intraoperative and local anesthesia has been administered. Acetaminophen is effective in treating postoperative pain post-DCR surgery in most patients. It reduces the need for opioid analgesia, has a favorable side effect profile, and does not affect bleeding. Opioid analgesia is rarely if at all necessary. Antiemetics tend to be rarely necessary with total intravenous anesthesia but should be available, to avoid precipitating bleeding.
External DCR
External DCR can be performed under general anesthesia (GA) or local anesthesia (LA) with some sedation. It is essential to remember that some blood will accumulate in the patient's nasopharynx, and airway protection (either self or via an endotracheal tube with cuff or throat pack) is important to prevent aspiration. If general anesthesia is used, it is useful for the blood pressure to remain in the slightly hypotensive range, as this aids hemostasis. For local anesthesia of the nasal mucosa, oxymetazoline spray, Moffett's solution, or cocaine paste/drops may be used. These help to both decongest and aid with hemostasis. A useful technique is to soak neurosurgical patties or ribbon gauze and gently pack the nose, including the middle meatus, above the middle turbinate, anterior to the middle turbinate, and on the nasal septum under direct visualization.
For local anesthesia of the skin, any product such as bupivacaine 0.5% with 1:200 000 epinephrine can be used. We like to perform a medial peri-bulbar block, along with infraorbital nerve block and subcutaneous injection for adequate analgesia. Care should be taken to not pierce the angular vein during LA injection, to avoid hematoma formation. Once the nasal mucosa is visible intraoperatively, further LA can be injected under direct visualization to provide analgesia and hemostasis.
Endoscopic DCR
The authors perform endoscopic DCR under general anesthesia, although other surgeons use sedation anesthesia. Anesthetic personnel should be aware of the possible accumulation of blood in the nasopharynx, and appropriate precautions should be implemented to avoid aspiration. The nasal mucosa requires decongestion for adequate visualization and should be prepared as described above. Further, hemostasis and analgesia are achieved with injection of 2 ml of lidocaine 2% with 1:100,000 epinephrine, using a dental syringe above and anterior to the middle turbinate under endoscopic visualization as part of the procedure.
Personnel
Only surgeons adequately trained in the relevant procedure, and with an understanding of the anatomy should attempt DCR surgery. A competent scrub nurse trained in the set up of endoscopic equipment is invaluable, and a good anesthetist can contribute considerably to a relatively bloodless surgical field.
Treatment / Management
Vasoconstriction and Hemostasis
Vasoconstriction is essential in order to control any hemorrhage, especially in the setting of endoscopic DCR.
Various measures can be employed in the peri-operative period to reduce bleeding:
- Adequate nasal preparation of the nose (as described above) regardless if LA or GA is used.
- Reverse Trendelenburg position
- If the patient is under GA, they should be maintained at slight hypotension.
- Tranexamic acid may be given intraoperatively, but care must be taken in patients with significant renal impairment or recent thromboembolic events.
- If external DCR is performed under LA, the orbicularis dissection should be performed gently using blunt dissection only. Once the bony osteotomy has been made, the nasal mucosa can be infiltrated with further LA, aiding both analgesia and hemostasis.
External DCR
- The face and nose are prepared with a standard surgical skin preparation. Chlorhexidine skin preparation is preferred.
- When draping a patient under LA, the entire face may be left exposed. When the patient is under GA, the endotracheal tube should be covered with the drape, leaving good access to the nose.
- One dose of intravenous broad-spectrum antibiotic is recommended prior to skin incision.
- While there are a variety of skin incisions, a straight incision using a number 15 blade from just above the MCT (about 2mm) to approximately 15 mm below is recommended, taking care to avoid the angular artery.
- The skin flap is then gently dissected off orbicularis.
- Orbicularis muscle is dissected off the MCT using blunt dissection. Cotton tipped applicators work very well, or blunt-tipped scissors may be used.
- The junction of the preseptal and pretarsal orbicularis fibers at the bony attachment of the MCT is relatively avascular and should be divided here.
- A number 15 blade or Rollett's rongine is then used to cut or disinsert the anterior limb of the MCT, and the Rollett's rongine is then used to cut the periosteum down the anterior lacrimal crest.
- The periosteum can be peeled away until the entire lacrimal fossa is exposed.
- A right-angled periosteal elevator is used to perforate the thin bone between the sac and anterior ethmoids along the suture line.
- The same right-angled periosteal elevator is placed within the perforated bone and turned gently to lift away the nasal mucosa, in order to avoid catching it during the creation of the osteotomy.
- Using a Kerrison rongeur, a bony ostium is created. This should extend:
- Superiorly to just above the MCT
- Anteriorly, to 10 to 15 mm anterior to the lacrimal crest
- Inferiorly to the nasolacrimal duct.
- The sac can then be probed using a "00" probe via the lower punctum.
- With the lacrimal sac tented, an "H" shaped incision is made. This may be made with a cataract surgery keratome, a number 11 blade, or Wescott scissors. It is essential to avoid damage to the internal ostium at this point and open the sac fully. Any stones can be removed, and biopsies may be taken as needed.
- The internal ostium is then inspected. If a membranectomy is required, this can be performed at this stage.
- Another "H" shaped incision is placed in the nasal mucosa. Care should be taken to match the shape and length of the mucosal flaps to each other to avoid excision of tissues.
- If bicanalicular or monocanalicular tubes are being used, these can be placed at this point.
- The flaps are then sutured to one another, with the posterior flaps being approximated first using polyglactin 910 suture. For this step, it is advantageous to have a half circle need as this assists greatly with the passage of the suture. Once the tubes (if used) are placed in the nose, the anterior flaps can be sutured in the same fashion. When suturing the anterior flaps, a small amount of orbicularis may be captured in the same bite.
- The MCT can be sutured into its approximate position using the same suture.
- The orbicularis should not be closed separately to avoid scarring.
- The skin should be closed with a non-absorbable suture. We prefer 6.0 polypropylene, passed in a continuous subcutaneous fashion.
- Steristrips are placed over the wound.
Useful Tips
- Ensure good anesthesia and hemostasis.
- After the initial incision, raise the skin flap and work between orbicularis and skin to avoid damaging the angular vein.
- Orbicularis fibers can be divided with blunt dissection using cotton buds.
- Avoid orbicularis sutures to avoid an unsightly scar.
- A back cut towards the upper eyelid can be made perpendicular to the upper edge of the incision when the patient's skin is thin and prone to tearing.
- An adequate bony ostium is essential to ensure long term success.
Endoscopic DCR
- The face and nose are prepared with a standard surgical skin preparation. Chlorhexidine skin preparation is preferred.
- When draping, the endotracheal tube should be covered with the drape, leaving good access to the nose.
- One dose of intravenous broad-spectrum antibiotics is recommended.
- The nose should be prepared with a decongestant, as described in the anesthesia section.
- The surgeon should familiarize themselves with the individual anatomy, including the roof of the nose, the location of the middle turbinate and axilla as well as the inferior turbinate using the endoscope (a 30-degree angle is preferred).
- Local anesthetic such as 2% lidocaine with 1: 800 000 adrenaline in a dental syringe is infiltrated into the lateral nasal wall above and in front of the middle turbinate. For easier infiltration, it is useful to bend the needle at a 30-degree angle, approximately 1 cm from the tip. This LA will provide both analgesia and hemostasis.
- The initial mucosal incision is critical and can be performed with a number 15 blade, but a crescent blade is preferred, used routinely in corneal surgery, such as a small-angled crescent blade.
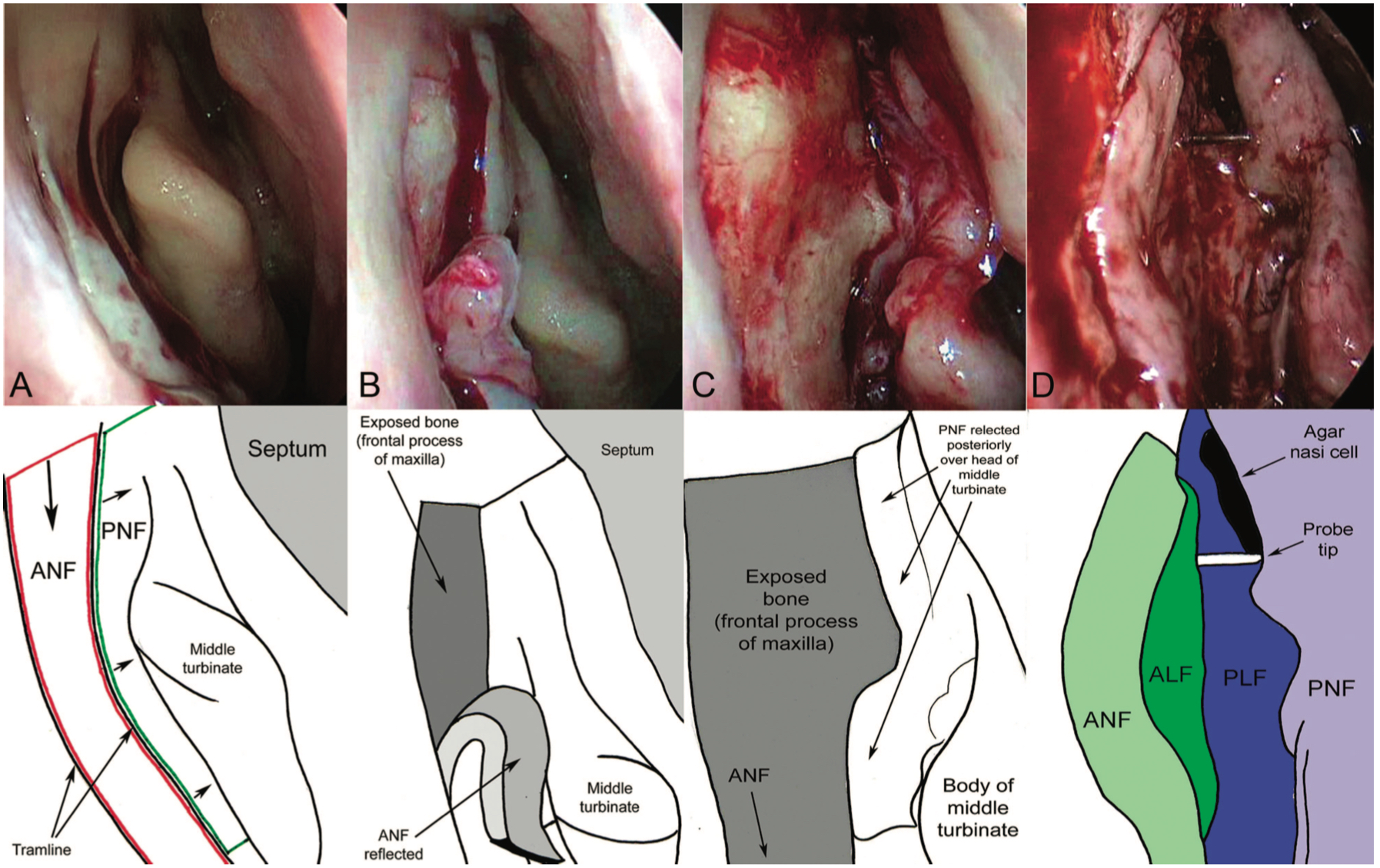
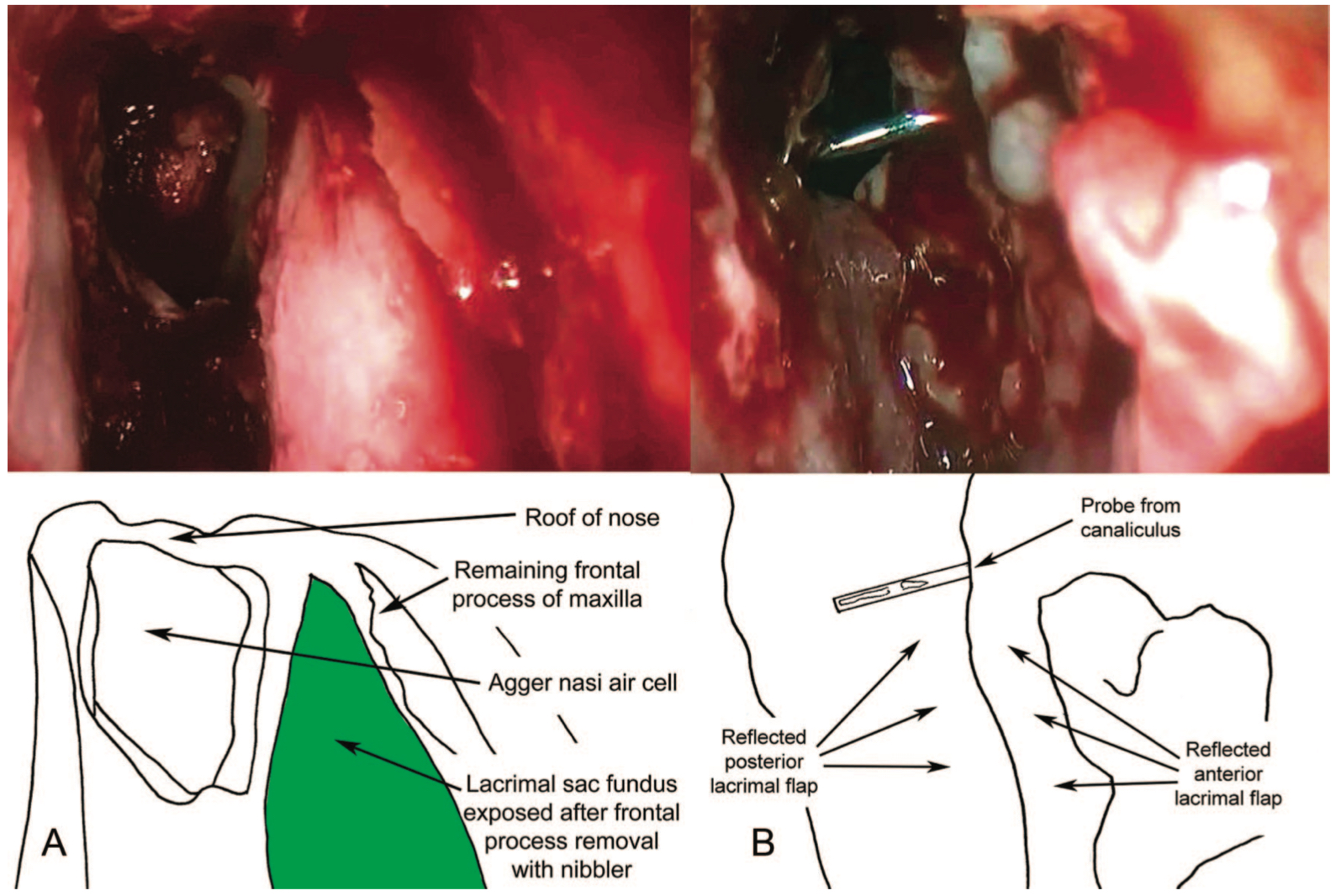
- The incision is created as 2 long parallel cuts: One mucosal incision is made on the lateral nasal wall approximately 3 mm posterior to the maxillary line, starting 8 mm above the insertion of the middle turbinate and extending vertically down to a level just below the body of the middle turbinate. A parallel vertical incision is made 8 mm anterior to this, and the 2 were joined superiorly by using a no. 15 scalpel blade. (Fig 2 Mucosal flaps)
- A back cut / relieving cut is then made from the inferior edge of the posterior cut, backward into the nose.
- The mucosal flap is then elevated from the underlying bone using suction Freer. The flap should be peeled inferiorly and placed "on the floor" to avoid catching it during the procedure. The mucosa posteriorly is peeled off slightly to enhance access.
- The bone should now be exposed, and a Kerrison punch can be used to enter the lacrimal bone. Using both Kerrison punch and Malhotra nibbler, the bone should be removed to above the middle turbinate as far towards the roof as possible, as only this thorough removal reduces the risk of surgical failure.
- Anteriorly the osteotomy should extend to the border of the flap created. Note that orbicularis fibers may be caught if bone removal extends too anteriorly and can cause a hematoma. Any sudden sheen or glistening of the view may be indicative of the breach of orbital fat, and the position of the instruments needs to be re-evaluated.
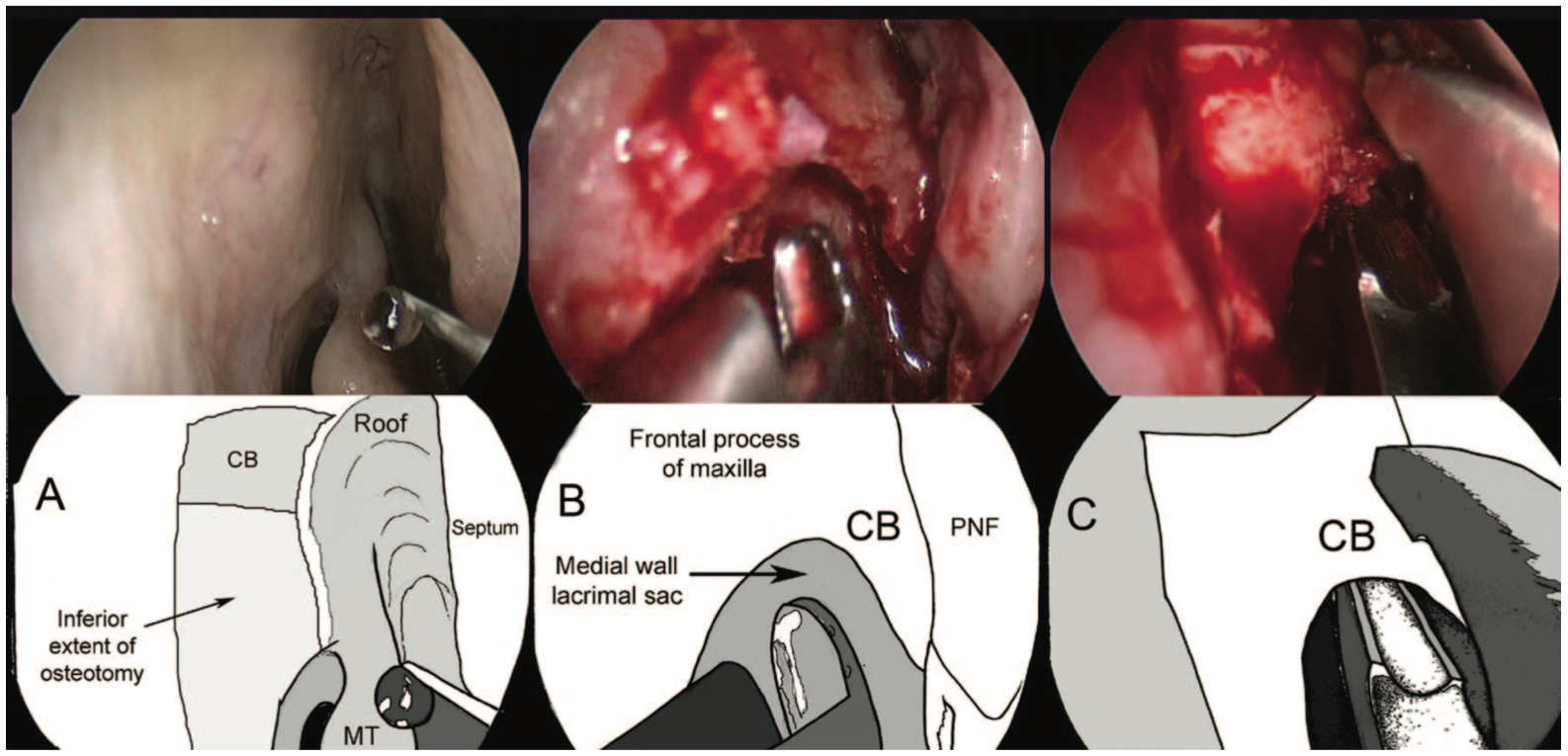
- At this point, some surgeons prefer to use a powered drill; however, most feel that manual instruments are sufficient. (Fig 3- Osteotomy creation)
- Once the entire lacrimal sac is exposed, a "00" probe should be placed via the lower punctum. This tents the sac, and the need for further bone removal can be visualized.
- The lacrimal sac flaps are then created by using a crescent blade, starting from inferiorly, cutting in an upward fashion. Care should be taken to avoid damage to the internal ostium. Once the entire lacrimal sac has been incised vertically, "I" shaped flaps superiorly and inferiorly are made to open the sac.
- At this stage, the surgeon must observe the quality of the internal ostium, along with the sac mucosa. Biopsies may be taken at this point, and dacryoliths can be removed. If the sac mucosa is thickened due to chronic inflammation, multiple radial cuts are often required to lie the flaps flat. (Fig 4- Probe within internal ostium, sac flaps opened)
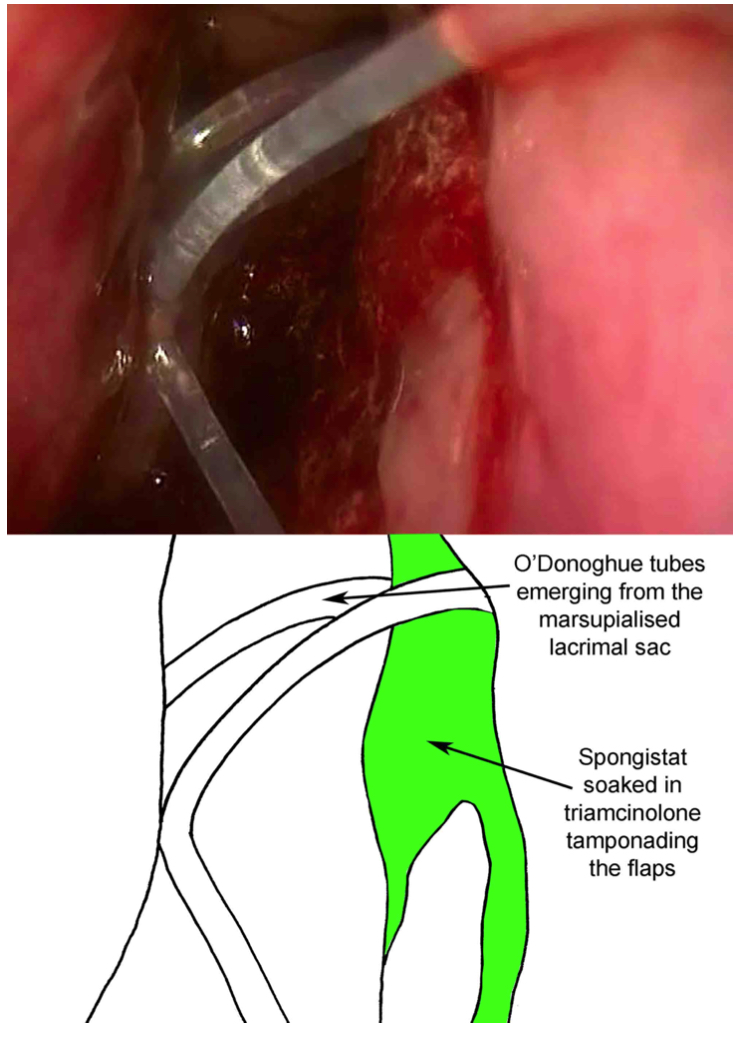
- Monocanalicular or bicanalicular tubes can then be placed via the puncta. (Fig 5- Placement of O'Donoghue tubes)
- The nasal mucosa flap is then repositioned, and care should be taken to approximate the nasal mucosa to the sac flaps.
- Alginate hemostat/gel foam is cut up in small squares, approximately 3x3 mm, then soaked in triamcinolone and placed into the nose. Care should be taken to cover the internal ostium, the sac flaps, and all bleeding areas (including septum) in order to minimize postoperative bleeding and epistaxis.
- The pharynx should then be suctioned via the inferior meatus prior to the cessation of the case.
Useful Tips
- Ensure good anesthesia and hemostasis.
- The bony ostium must be sufficiently sized to avoid surgical failure.
- Care must be taken to avoid touching the internal ostium during the creation of sac flaps. Any trauma may contribute to scarring and subsequent surgical failure.
- Should excessive bleeding occur intra-operatively, it is useful to pack the nose with neurosurgical patties soaked in Moffett's solution or adrenaline. Warm water irrigation may also help.
- Generous use of alginate-based dressing at the end of the procedure reduces postoperative epistaxis.
Notes on Equipment Used in Endoscopic DCR
Mechanical instruments or a powered drill may be used for the creation of the bony ostium. As emphasized previously, especially the superior bone removal is important. While powered instruments such as diamond burrs have been used widely and yield good results, the use of a bone nibbler is associated with lower cost and possibly quicker operating time.[32][7] It allows effective removal of the superior bone. As described by Patel et al., it is important to avoid twisting motions for the most superior aspect of bone removal and employ a crushing motion instead, in order to avoid a spiral base of skull fracture. The angled anterior aspect of the bone nibbler makes it ideal for removing the most superior bone to create a large ostium. Despite the bone being thicker in people of Asian descent, there have usually been no problems with removing this manually, when utilizing the crushing technique (Malhotra, personal correspondence).[18] It may be more cost-effective on a long term basis to use this device, as it can be resterilized and requires no disposable parts or additional machines or equipment.
Pediatric DCR
Pediatric DCR is a safe procedure with high success rates (over 90%) for congenital and acquired nasolacrimal duct obstruction.[33] Success rates are slightly lower for children with canalicular pathology and craniofacial anomalies or Down syndrome.
Both endonasal and external DCR may be used.
- Most cases of congenital NLDO resolve spontaneously by age 1.
- Patients should have undergone previous syringing and probing +/- intubation or balloon dilation in order to be considered for DCR.
- Patients should only undergo surgery once they have been deemed to be safe from an anesthetic point of view. It is essential to recall that children with Down syndrome may have undiagnosed cardiac abnormalities and more difficult airways. Children with craniofacial abnormalities may pose a considerable anesthetic challenge. Prematurely born children may only be considered for surgery once they have achieved at least 12 months of corrected age.
- As babies and some toddlers are obligate nasal breathers, DCR should only be performed one side at a time.
- The skull base in children may also be slightly lower than in adults. The nasal space is overall much narrower than in adult endo DCR, which is mostly an issue for children under 2 years but resolves by the time they reach school age.
- Pediatric patients require a high heart rate in order to maintain their cardiac output, which makes intraoperative bleeding more likely.
- Total intravenous anesthesia (TIVO) may create a drier surgical field and less bleeding.
- Nasal packing: Cocaine is contra-indicated in children and should be avoided. Oxymetazoline and/or epinephrine-soaked gauze or neuro-patties may be used, depending on the anesthetic preference.
- Pediatric endoscopic DCR should only be attempted by surgeons comfortable with adult endoscopic DCR.
Use of Lacrimal Intubation in DCR
Lacrimal intubation or transcanalicular stenting is commonly performed, assuming that the stenting prevents membranous occlusion or fibrotic stenosis of the internal ostium. Stents may have both functional and mechanical effects. On a functional level, the surface tension of the silicone stent allows the flow of tears along the periphery of the stent. The stent also mechanically dilates the lumen and straighten canalicular bends – therefore causing better flow through these narrow conduits.
A large variety of materials (synthetic, metal, and organic) have been used as stents for stenotic puncta or canaliculi. These include silk, nylon, dacron, silver wires, malleable metal, polyethylene, and polypropylene. Stent design has evolved in an ever-continuing effort to improve success rates and to reduce complication rates. The ideal stent should be simple to use, widely available, affordable, inert, pliable, self-retaining, and cause minimal damage to surrounding tissue. Silicone, which holds many of these properties, is the commonest material used in stents.
Transcanalicular stents can be classified into monocanalicular and bicanalicular stents. Monocanalicular stents usually traverse a single canaliculus to the lacrimal sac and sometimes to the NLD, while bicanalicular stents pass via both canaliculi to the sac and nasal cavity where they are tied in a loop. Bicanalicular stents are made from silicone with blunt steel guides that are used to intubate the nasolacrimal system. Monocanalicular stents are increasingly utilized in DCR surgeries due to their ease of insertion and removal. Detorakis et al. showed monocanalicular intubation using mini-Monoka in external DCR to be safe and effective.[34]
Monocanalicular stents have the advantage that only one patent canaliculus is required for their usage. Insertion and removal are relatively straightforward, and their stable anchoring mechanism in the punctum avoids bothersome protrusion or cheese wiring.
Bicanalicular stents address both canaliculi and are secured on both ends of the lacrimal system and are therefore less prone to migration or extrusion. They may also function as a mechanical barrier to prevent the closure of the internal ostium and keep the soft ostium between the sac and nasal cavity patent during DCR healing. It is, however, important to remember that DCR surgery is more likely to fail if the bony ostium is too small. Canalicular intubation will not be able to counteract an insufficiently sized ostium regardless of the type of stent utilized.
There are no well-established indications or guidelines for canalicular intubation in DCR. Definitive canalicular pathology is an evidence-based indication for lacrimal intubation during DCR surgery. Patients with previous DCR and anatomic success but functional failure appear to benefit from intubation, as do patients with canalicular obstruction.[35][36] Other possible indications for canalicular intubation generally lack evidence and include previous dacryocystitis, poor flap creation, revision surgery, excessive bleeding, inflammatory disease (e.g., sarcoidosis), and small lacrimal sacs.
When endoscopic DCR surgery is performed by less experienced or trainee surgeons, there is likely to be more mucosal trauma, which can increase the chance of scarring and failure. These patients, therefore, appear to benefit from intubation.
A recent randomized trial compared the outcomes after endoscopic DCR after 12 months with and without silicone tubes.[37] Patients were randomized to receive lacrimal intubation or not. Three hundred patients had 12 months of follow-up. Success was defined as both anatomical and functional success, and overall success, both subjectively and objectively, was 94.7% in the stented group and 87.8% in the non-stented group. The authors found that the failure rate was more than twice as high when tubes were not used (12.2% vs. 5.3%). This study was both methodological sound and had appropriate follow up times, it is the largest randomized trial to date.
No clear data exists on the timeframe for the removal of the lacrimal stents in the postoperative period. While some surgeons choose to remove lacrimal stents after 3 months, and others advocate earlier removal after around 4 weeks.
Complications of lacrimal stents depend upon their type: bicanalicular tubes can cheese wire or prolapse, each of which occurs in approximately 4% of patients.[37] These are the most common complications, but others can include ocular surface irritation and stent incarceration. As monocanalicular tubes often contain a phalange stabilizing one end within the punctum, prolapse is rare. It is potentially possible for the patient to remove the tube with vigorous eye rubbing. If the punctum is large such as following a previous punctoplasty, the stent may become dislodged into the canaliculus, but migration may also occur spontaneously in children.
Differential Diagnosis
- Acute complications of sarcoidosis
Complications
Risks Common to All Types of DCR
- Bleeding
- Infection
- Complications related to silicone stent usage, including injury to lacrimal punta
- Failure to improve tearing
- Cerebrospinal fluid (CSF) leak, presumably due to a spiral fracture, has been reported to occur as a rare complication of endoscopic DCR and is well established as a rare risk during external DCR.[38] To further reduce the risk, it is advisable to avoid twisting movements when removing superior bone during intraoperative bony ostium creation, as this could create a spiral fracture into the base of the skull and cribriform plate.
Risks: External DCR
- External DCR will always have a scar at the location of the skin incision. This is noticeable in approximately 1 in 5 patients and cosmetically significant in 1 in 10.[39]
- Facial nerve injury also is common during external DCR and can cause variable lagophthalmos. While this is temporary in most patients, it can take up to 30 weeks to recover, but it also may remain permanent in 1 to 2%.[40] This occurrence of blink-lagophthalmos and medial orbicularis weakness may explain the concept of occasional “pump-failure” following external DCR.
Risks: Endoscopic DCR
- Damage to the nasal mucosa with adhesion formation
- Orbital fat prolapse,
- Injury to the medial rectus muscle. This is rare and only occurs if the location of the sac is unclear to the operating surgeon, and bone removal with breach of the periorbita is performed posterior to the lacrimal sac.
Postoperative and Rehabilitation Care
- The patient should be observed for epistaxis in the immediate postoperative period. Some mild epistaxis is common and usually ceases after 12 to 24 hours.
- If the patient develops significant epistaxis, the usual resuscitation measures should be instigated. Immediate digital pressure should be applied over the anterior nose for at least 10 minutes. The nose can then be packed firmly with either gauze or an epistaxis pack. A return to the operating theatre should be considered with ongoing hemorrhage.
- In external DCR cases, the wound should be kept dry, and the sterile strips skin closures intact.
- Hot drinks should be avoided during the initial 48 hours, as they can contribute to epistaxis.
- The patient should avoid blowing the nose for the first week and sneeze with the mouth open.
- After any epistaxis has ceased (usually day 1), the patient may commence a steroid-based spray such as fluticasone nasal spray morning and night for 4 weeks.
- Topical or systemic antibiotics are not required.
- In external DCR, skin sutures should be removed after 7 to 10 days.
- If silicone intubation has been performed intraoperatively, the tubes can be removed 3 to 4 weeks postoperatively, although some surgeons remove them three months after surgery.
Deterrence and Patient Education
- Preoperative cessation of anticoagulants for 7 to 10 days prior to surgery should be discussed with the patient.
- The surgical technique being used should be discussed with the patient using a handout or videos.
- The success and failure rate of the chosen procedure and also based upon the surgeon's experience should be discussed.
- Postoperative care of the wound is discussed.
- Avoidance of blowing the nose for one week after surgery is discussed.
Pearls and Other Issues
- The DCR surgeon should aim to use the same gauge lacrimal cannula and same sized syringe in the clinic when irrigating the lacrimal system, to develop “a feel” of what is normal saline passage into the nose.
- Excellent knowledge of lacrimal, eyelid, and nasal anatomy is needed to perform DCR successfully.
- One-on-one training is essential to learn the endoscopic approach.
- The surgeon must become familiar with methods of controlling bleeding from the nose after DCR.
Enhancing Healthcare Team Outcomes
Only surgeons adequately trained in the relevant procedure and with an understanding of the anatomy should attempt DCR surgery.
A competent scrub nurse trained in the set up of endoscopic equipment is invaluable and a good anesthetist can contribute considerably to a relatively bloodless surgical field. [Level 3]