Introduction
The cranial nerves primarily innervate the head and neck structures. Unlike spinal nerves, which originate from neural fibers in the spinal grey matter, cranial nerves consist of neural processes associated with specific brainstem nuclei and cortical structures. Additionally, cranial nerve nuclei are functionally organized within the brainstem, with sensory nuclei typically positioned more posteriorly and laterally, while motor nuclei are more anterior.
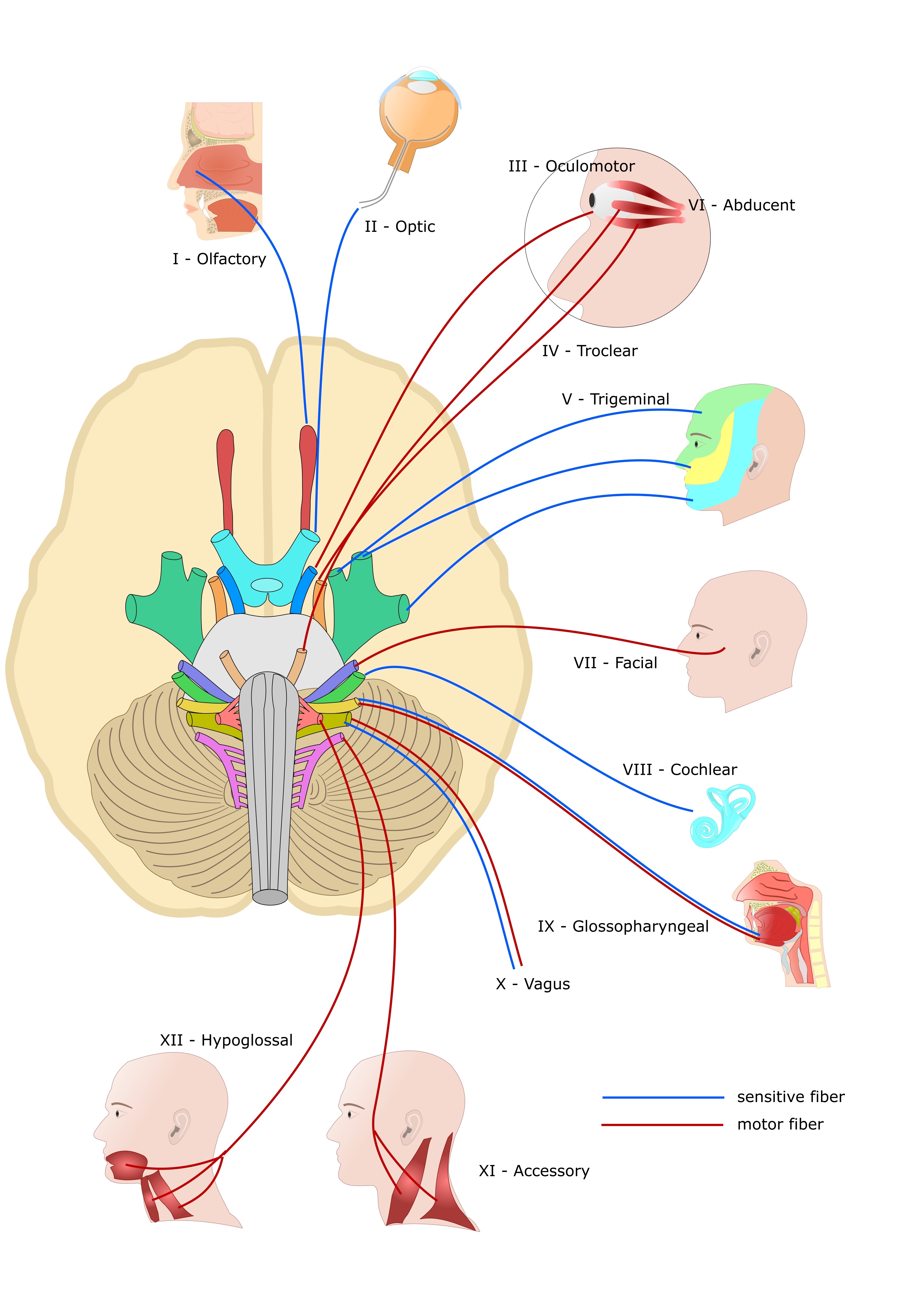
Cranial nerves I (olfactory), II (optic), and VIII (vestibulocochlear) are purely sensory. Meanwhile, cranial nerves III (oculomotor), IV (trochlear), VI (abducens), XI (spinal accessory), and XII (hypoglossal) are purely motor. The remaining cranial nerves—V (trigeminal), VII (facial), IX (glossopharyngeal), and X (vagus)—are mixed, carrying both sensory and motor fibers (see Image. Cranial Nerves).[1]
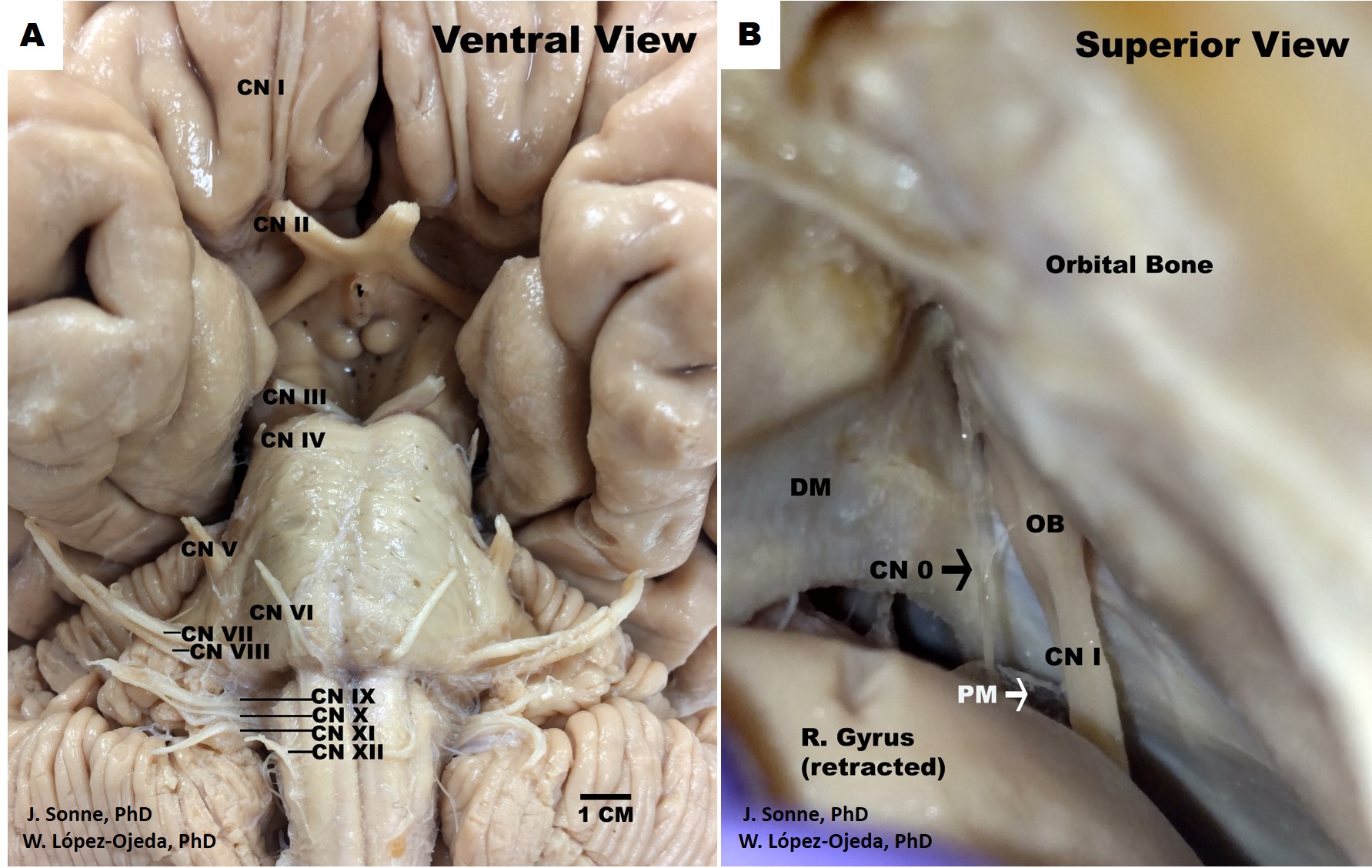
While this classification represents the traditional way of organizing and indexing cranial nerves, the scientific reality is more complex. Ongoing debate persists in the academic community regarding the classification, routes, and identification of distinct cranial nerve fibers. Additionally, less recognized structures, such as the terminal nerve—also referred to as cranial nerve 0 or nerve nulla—have been proposed.[2] Following the traditional framework, the terminal nerve could be classified as a purely sensory nerve.
Damage to cranial nerves, their tracts, or nuclei typically results in well-defined clinical syndromes. The systematic assessment of cranial nerves is central to neurological diagnosis, offering insight into the integrity of the central and peripheral nervous systems.
Structure and Function
The cranial nerves are typically described either by their anatomical numbering from I to XII, reflecting their sequential origins from the caudal to the ventral brainstem, or by grouping them based on their developmental functions, whether sensory, motor, or mixed. In this section, we first outline the 12 cranial nerves in their anatomical order, followed by a brief overview of their functional groupings (see Image. Human Brain Cranial Nerves).
Cranial Nerve I (Olfactory Nerve)
Special visceral afferent (SVA) bipolar sensory neurons reside in the olfactory mucosa, an area measuring approximately 2 to 4 cm² in the roof of the nasal cavity, spanning the superior nasal concha and nasal septum. These neurons are organized into nerve bundles known as filia olfactoria, which collectively form the olfactory nerve. The nerve fibers enter the cribriform plate of the ethmoid bone to synapse in the olfactory bulb, situated in the anterior cranial fossa.
The olfactory bulb represents the anterior-most part of the olfactory tract, situated at the inferior surface of the frontal lobe. This structure extends posteriorly between the orbital gyrus and the gyrus rectus, bifurcating at the olfactory trigone into the medial and lateral olfactory striae. These striae terminate in the higher-order cortex.[3] Some olfactory projections travel medially to the septal area and the contralateral bulb via the anterior commissure, while others travel laterally to the amygdala and piriform cortex—the primary olfactory cortex— where the conscious perception of odorants is processed.[4]
Cranial Nerve II (Optic Nerve)
Cranial nerve II, the optic nerve, transmits special somatic afferent (SSA) visual sensory information from the rods and cones of retinal sensory receptors to the thalamus, primarily the lateral geniculate nucleus and the superior colliculus. This nerve is not considered a true cranial nerve, as it represents an extension and evagination of the diencephalon. Ganglion cells in the retina form central projections that constitute the optic nerve fibers, which pass through the optic canal to enter the middle cranial fossa.
Fibers representing the medial visual fields travel posteriorly without crossing at the optic chiasm, while those representing the lateral visual fields decussate within the chiasm. This retinotopic organization continues within the optic nerve and its synapses in the lateral geniculate nucleus (LGN). Collateral fibers project centrally to the superior colliculus, which mediates the pupillary light reflex and connects to the pulvinar of the thalamus. These pulvinar connections contribute to the phenomenon of blindsight.[5] These pulvinar collaterals enable cortically blind individuals to make unconscious eye movements and detect light direction within the visual field.[6][7][8] From the LGN, axons project to the visual cortices in the occipital lobes for conscious visual processing.
Cranial Nerves III, IV, and VI (Oculomotor, Trochlear, and Abducens Nerves)
Cranial nerves III, IV, and VI—the oculomotor, trochlear, and abducens nerves, respectively—are general somatic efferent (GSE) nerves that innervate the extraocular muscles within the orbit (see Image. Orbit, Anterior View). These nerves travel ipsilaterally from their respective brainstem nuclei through the calvarium and enter the orbit via the superior orbital fissure.
The oculomotor nerve passes through the common tendinous ring, which serves as the shared attachment point for the 4 rectus muscles and the abducens nerve. The trochlear nerve, in contrast, enters the orbit outside the common tendinous ring to innervate the superior oblique muscle. The abducens nerve specifically innervates the lateral rectus muscle, and its function can be assessed by testing the ability of the eye to abduct.
Cranial nerve III innervates most of the eye muscles by splitting into superior and inferior branches, which innervate the remaining 3 rectus muscles, the inferior oblique, and the skeletal muscle component of the levator palpebrae superioris. Besides its GSE function, cranial nerve III also contains a general visceral efferent (GVE) component originating from the Edinger-Westphal nucleus, also known as the accessory or visceral oculomotor nucleus. These parasympathetic fibers travel with the oculomotor nerve to synapse in the ciliary ganglion within the orbit.
The postganglionic parasympathetic fibers from the ciliary ganglion pass through the sclera to innervate the pupillary sphincter and the ciliary smooth muscles, responsible for pupillary constriction and lens accommodation. The pupillary light reflex, involving efferent pathways from the superior colliculus to the accessory oculomotor nucleus, may be used to test pupillary constriction.[9] The eye movement test, including abduction, adduction, infraduction, and supraduction, effectively evaluates the function of the GSE components of cranial nerves III, IV, and VI.
Cranial Nerve V (Trigeminal Nerve)
Cranial nerve V, the trigeminal nerve, is responsible for the general somatic afferent (GSA) or sensory innervation of the face through its 3 main branches: V1 (ophthalmic), V2 (maxillary), and V3 (mandibular). Additionally, cranial nerve V, via its V3 branch, provides motor innervation (special visceral efferent or SVE) to the muscles of mastication, as well as the anterior belly of the digastric muscle, mylohyoid, and 2 small tensor muscles, the tensor veli palatini and tensor tympani.
Although cranial nerve V does not carry autonomic fibers as it exits the pons, parasympathetic fibers from other mixed cranial nerves join its peripheral branches to innervate target structures such as the lacrimal, parotid, submandibular, and sublingual glands. Consequently, while central nuclear or supranuclear lesions can lead to ipsilateral sensory or motor deficits, peripheral nerve damage to specific branches primarily affects parasympathetic functions.[10]
Cranial Nerve VII (Facial Nerve)
Cranial nerve VII, the facial nerve, contains motor, autonomic, and minor somatosensory fibers. The SVE fibers innervate the muscles of facial expression and exit the skull through the stylomastoid foramen, deep to the parotid gland. Damage to these fibers results in ipsilateral facial paralysis, also known as facial palsy.
GVE and SVA fibers initially exit the brainstem as the nervus intermedius. This separate nerve bundle merges with the other components of the facial nerve within the facial canal. The GVE fibers from the superior salivary nucleus provide parasympathetic innervation to the glands and mucosa of the face, excluding the parotid gland and smaller buccal and labial glands. Taste fibers from the anterior 2/3 of the tongue travel as the chorda tympani nerve, which synapses in the solitary nucleus after passing through the geniculate ganglion. Damage to the facial nerve can also affect these visceral components, depending on the location of the lesion. GSA fibers provide sensory innervation from the auricle and a small external portion of the auditory canal.[11]
Cranial Nerve VIII (Vestibulocochlear Nerve)
Cranial nerve VIII, the vestibulocochlear nerve, is responsible for both auditory and vestibular sensations related to head orientation. This nerve conveys SSA fibers from the inner ear to the cochlear and vestibular nuclei located in the caudal medulla oblongata. Sensory receptor cells called "hair cells," found in the cochlear duct, semicircular canals, utricle, and saccule, have apical ciliary extensions that transduce mechanical deformation into an electrochemical signal. Ganglionic neurons in the cochlea and vestibular system receive this signal peripherally and transmit it centrally through the internal auditory meatus before entering the medulla.[12]
Cranial Nerve IX (Glossopharyngeal Nerve)
Cranial nerve IX, the glossopharyngeal nerve, provides SVE innervation to the stylopharyngeus and pharyngeal constrictor muscles via the nucleus ambiguus. Fibers from the inferior salivary nucleus travel with cranial nerve IX to provide GVE innervation to the parotid, buccal, and labial glands. Visceral afferents, including both general visceral afferent (GVA) and SVA fibers, transmit sensory information from the carotid body and carotid sinus, as well as taste from the posterior 1/3 of the tongue to synapse in the solitary nucleus. Additionally, GSA fibers convey sensory information from the skin over the tongue, the oropharynx, the middle ear cavity, and the auditory canal.[13]
Cranial Nerve X (Vagus Nerve)
Cranial nerve X, the vagus nerve, carries parasympathetic efferent GVE fibers from the dorsal vagal nucleus to the thoracic and abdominal viscera, extending as far as the splenic flexure of the colon. These fibers form an extensive plexus that travels along the esophageal serosa to the viscera. The vagus nerve also has significant SVE output via the nucleus ambiguus to the pharyngeal and soft palate muscles, as well as the intrinsic laryngeal muscles through the superior and recurrent laryngeal nerves.
GSA fibers provide sensory input from the posterior cranial dura and part of the ear and external auditory canal epithelium. GVA fibers transmit sensory information from the pharynx, larynx, aorta, thoracic and abdominal viscera, and taste buds at the root of the tongue and epiglottis SVA, all of which synapse in the solitary nucleus. Damage to the recurrent laryngeal branch of the vagus nerve can result in vocal hoarseness or acute dyspnea, particularly with bilateral avulsion.
Cranial Nerve XI (Accessory Nerve)
Cranial nerve XI, the spinal accessory nerve, provides GSE motor innervation to the trapezius and sternocleidomastoid muscles via the spinal nucleus of the accessory nerve. This nucleus is located within the cervical spinal cord, spanning from the levels of C1 to approximately C5 or C6. The fibers emerge as independent roots, separate from the anterior or dorsal spinal roots of the central spinal grey matter, and ascend through the foramen magnum to enter the cranial cavity. From there, these fibers exit the cranial cavity via the jugular foramen alongside cranial nerves IX and X.
Cranial Nerve XII (Hypoglossal Nerve)
Cranial nerve XII, the hypoglossal nerve, provides GSE innervation to the intrinsic and extrinsic muscles of the tongue, excluding the palatoglossus muscle. The supplied muscles include the genioglossus, geniohyoid, hyoglossus, and styloglossus muscles. Fibers from the hypoglossal nucleus exit the medulla at the sulcus between the pyramids and the olives as a bundle, which coalesces before entering the hypoglossal canal to exit the cranium.[14]
The cranial nerves may also be grouped based on their functional roles: efferent (motor output), afferent (sensory input), or mixed. Cranial nerves I, II, and VIII are purely afferent, as they transmit sensory information from the olfactory region, the retina of the eye, and the inner ear, respectively. Cranial nerves III, IV, VI, XI, and XII are purely efferent, as they provide motor output to the orbit, neck, and tongue. Cranial nerves V, VII, IX, and X are mixed, containing both afferent and efferent fibers responsible for sensory and motor functions.
Clinical Significance
Cranial Nerve I
Traumatic injury, especially “whiplash” from automobile collisions, can sever the olfactory projections passing through the cribriform plate, leading to anosmia. This loss of smell has been linked to the development of depression.[15][16][17] The sense of olfaction also seems to play an unconscious role in activating the limbic system, which may help explain this association.[18]
Cranial Nerve VIII
Damage to the vestibular component of this nerve causes dizziness, while damage to the cochlear component leads to peripheral or sensorineural hearing loss. The internal auditory meatus, a narrow canal in the temporal bone, serves as the pathway for these nerves. Schwannomas of the vestibular or cochlear nerves within this meatus can compress and impinge the nerves. Early signs include progressively worsening hearing loss, tinnitus, and imbalance, often accompanied by a sense of pressure in the ear and facial weakness or paralysis.[19] Vestibular schwannomas have an incidence rate of 6 to 9 new cases per million people annually and are generally treatable with surgery or radiation.[20] However, if left untreated, these tumors can grow significantly and become life-threatening.
Cranial Nerve XI
Damage to the central root or nucleus of the spinal accessory nerve results in ipsilateral flaccid paralysis of the sternocleidomastoid, leading to difficulty turning the head against force, and partial ipsilateral paralysis of the trapezius, causing shoulder drop.[21][22] The trapezius is innervated by both the spinal accessory nerve and anterior horn grey matter from the cervical spinal regions C3 through C4 or C5. Thus, complete paralysis of the trapezius muscle does not typically occur from a simple focal lesion.[23]
Cranial Nerve XII
Damage to the nucleus or nerve fibers of the hypoglossal nerve causes the tongue to deviate toward the side of the lesion. As the ipsilateral genioglossus muscle weakens or becomes flaccid, its ability to protrude the tongue is reduced.
Other Issues
Cranial nerve 0, also known as the terminal nerve or nervus terminalis due to its proximity to the lamina terminalis, was first identified as a separate cranial nerve in humans in 1914. Nevertheless, this nerve is often overlooked in current anatomical textbooks. Cranial nerve 0 consists of an independent central plexus of small, unmyelinated fibers (possibly SVA fibers) located medially and close to the olfactory tract near the olfactory trigone. This nerve's discrete positioning may contribute to its poor identification during standard dissection techniques. The fibers of cranial nerve 0 travel centrally to subcortical structures, sending projections to areas such as the medial precommissural septum and the medial septal nucleus. Cranial nerve 0 appears to have a rich bundle of well-vascularized fibers that ascend from the nasal submucosa and project to key limbic structures, including the amygdala and hypothalamic nuclei.
Functionally, cranial nerve 0 is thought to play a role in the unconscious processing of pheromones by regulating autonomic responses through hypothalamic gonadotropin-releasing hormone (GnRH), possibly via the kisspeptin neuronal network. Clinically, disruption of the normal embryological migratory pathway of GnRH neural crest cells, originating from the olfactory placode and basal forebrain, can lead to Kallman syndrome. This genetic condition is characterized by hypogonadotropic hypogonadism with partial or total anosmia, and it results in abnormal sexual development in both sexes[24]
The canonical descriptions of the cranial nerves provide a broad overview of current medical literature. However, ongoing research continuously uncovers new findings, sparking debates regarding the original classification of some cranial nerve fibers. For example, researchers discovered that the nervus intermedius, traditionally associated with carrying taste fibers from the tongue as part of cranial nerve VII, also has visceral efferent connections with the vestibulocochlear nerve in the internal auditory meatus.[25] Additionally, various populations of efferent fibers to the organ of Corti hair cells modulate afferent transduction sensitivity, a feature present in mammals and other vertebrates.[26][27][28][29]
The classification of the cranial accessory nerve as a portion of either the vagus or accessory nerve remains a topic of ongoing debate. In most cases, the cranial accessory nerve, with fibers originating in the nucleus ambiguus of the medulla, has no connections with the spinal accessory nerve, which has fibers originating in the spinal accessory nucleus of the spinal cord.[30][31] Cranial nerve 0, recognized in human embryos since 1905 and in adults since 1914, was once included in historical textbooks, but modern anatomical texts have excluded it from the official list of cranial nerves.[32] However, the sensory function and role of this nerve in modulating the hypothalamic endocrine and autonomic systems make it physiologically and clinically relevant.
Similarly, some argue that the optic nerve should not be classified as a peripheral nerve but rather as a central cranial tract.[33] These examples highlight the ongoing evolution of anatomical and medical terminology and the structures they describe.[34][35] A holistic approach to cranial nerves may offer greater value than the categorical organization found in many textbook descriptions. These nuances merit attention, as understanding them is particularly important for medical and health professions education.