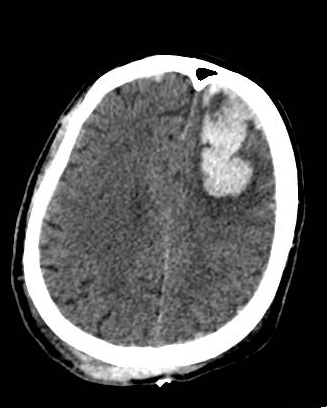
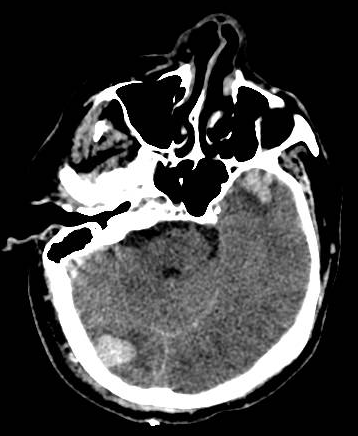
[1]
Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. The Journal of head trauma rehabilitation. 2010 Mar-Apr:25(2):72-80. doi: 10.1097/HTR.0b013e3181ccc8b4. Epub
[PubMed PMID: 20234226]
[2]
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, Wilberger JE, Surgical Management of Traumatic Brain Injury Author Group. Surgical management of acute subdural hematomas. Neurosurgery. 2006 Mar:58(3 Suppl):S16-24; discussion Si-iv
[PubMed PMID: 16710968]
[3]
McGinn MJ, Povlishock JT. Pathophysiology of Traumatic Brain Injury. Neurosurgery clinics of North America. 2016 Oct:27(4):397-407. doi: 10.1016/j.nec.2016.06.002. Epub 2016 Aug 10
[PubMed PMID: 27637392]
[4]
Lalwani S, Hasan F, Khurana S, Mathur P. Epidemiological trends of fatal pediatric trauma: A single-center study. Medicine. 2018 Sep:97(39):e12280. doi: 10.1097/MD.0000000000012280. Epub
[PubMed PMID: 30278499]
Level 2 (mid-level) evidence
[5]
Lu J, Marmarou A, Choi S, Maas A, Murray G, Steyerberg EW, Impact and Abic Study Group. Mortality from traumatic brain injury. Acta neurochirurgica. Supplement. 2005:95():281-5
[PubMed PMID: 16463866]
[6]
Nalliah RP, Anderson IM, Lee MK, Rampa S, Allareddy V, Allareddy V. Epidemiology of hospital-based emergency department visits due to sports injuries. Pediatric emergency care. 2014 Aug:30(8):511-5. doi: 10.1097/PEC.0000000000000180. Epub
[PubMed PMID: 25062295]
[7]
Schneider ALC, Wang D, Ling G, Gottesman RF, Selvin E. Prevalence of Self-Reported Head Injury in the United States. The New England journal of medicine. 2018 Sep 20:379(12):1176-1178. doi: 10.1056/NEJMc1808550. Epub
[PubMed PMID: 30231228]
[8]
Bullock R, Chesnut RM, Clifton G, Ghajar J, Marion DW, Narayan RK, Newell DW, Pitts LH, Rosner MJ, Wilberger JW. Guidelines for the management of severe head injury. Brain Trauma Foundation. European journal of emergency medicine : official journal of the European Society for Emergency Medicine. 1996 Jun:3(2):109-27
[PubMed PMID: 9028756]
[9]
Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. The Lancet. Neurology. 2008 Aug:7(8):728-41. doi: 10.1016/S1474-4422(08)70164-9. Epub
[PubMed PMID: 18635021]
[10]
Jiang JY, Chinese Head Trauma Study Collaborators. Head trauma in China. Injury. 2013 Nov:44(11):1453-7. doi: 10.1016/j.injury.2012.08.045. Epub 2012 Oct 13
[PubMed PMID: 23068139]
[11]
Parchani A, El-Menyar A, Al-Thani H, Tuma M, Zarour A, Abdulrahman H, Peralta R, Asim M, Latifi R. Recreational-related head injuries in Qatar. Brain injury. 2013:27(12):1450-3. doi: 10.3109/02699052.2013.823664. Epub 2013 Aug 7
[PubMed PMID: 23924056]
[12]
Leijdesdorff HA, van Dijck JT, Krijnen P, Vleggeert-Lankamp CL, Schipper IB, Regional Trauma Center West-Netherlands’ Research Group. Injury pattern, hospital triage, and mortality of 1250 patients with severe traumatic brain injury caused by road traffic accidents. Journal of neurotrauma. 2014 Mar 1:31(5):459-65. doi: 10.1089/neu.2013.3111. Epub 2013 Dec 21
[PubMed PMID: 24093437]
[13]
Fernández-Abinader JA, González-Colón K, Feliciano CE, Mosquera-Soler AM. Traumatic Brain Injury Profile of an Elderly Population in Puerto Rico. Puerto Rico health sciences journal. 2017 Dec:36(4):237-239
[PubMed PMID: 29220069]
[14]
Egbohou P, Mouzou T, Tchetike P, Sama HD, Assenouwe S, Akala-Yoba G, Randolph L, Tomta K. Epidemiology of Pediatric Traumatic Brain Injury at Sylvanus Olympio University Hospital of Lomé in Togo. Anesthesiology research and practice. 2019:2019():4038319. doi: 10.1155/2019/4038319. Epub 2019 Aug 1
[PubMed PMID: 31467523]
[15]
Cepeda S, Gómez PA, Castaño-Leon AM, Martínez-Pérez R, Munarriz PM, Lagares A. Traumatic Intracerebral Hemorrhage: Risk Factors Associated with Progression. Journal of neurotrauma. 2015 Aug 15:32(16):1246-53. doi: 10.1089/neu.2014.3808. Epub 2015 Apr 15
[PubMed PMID: 25752340]
[16]
Cepeda S, Castaño-León AM, Munarriz PM, Paredes I, Panero I, Eiriz C, Gómez PA, Lagares A. Effect of decompressive craniectomy in the postoperative expansion of traumatic intracerebral hemorrhage: a propensity score-based analysis. Journal of neurosurgery. 2019 Apr 26:132(5):1623-1635. doi: 10.3171/2019.2.JNS182025. Epub 2019 Apr 26
[PubMed PMID: 31026834]
[17]
Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. Journal of neurotrauma. 2012 Jan 1:29(1):19-31. doi: 10.1089/neu.2011.2122. Epub 2011 Dec 5
[PubMed PMID: 21988198]
[18]
Juratli TA, Zang B, Litz RJ, Sitoci KH, Aschenbrenner U, Gottschlich B, Daubner D, Schackert G, Sobottka SB. Early hemorrhagic progression of traumatic brain contusions: frequency, correlation with coagulation disorders, and patient outcome: a prospective study. Journal of neurotrauma. 2014 Sep 1:31(17):1521-7. doi: 10.1089/neu.2013.3241. Epub 2014 Jul 8
[PubMed PMID: 24738836]
[19]
Zhang J, Jiang R, Liu L, Watkins T, Zhang F, Dong JF. Traumatic brain injury-associated coagulopathy. Journal of neurotrauma. 2012 Nov 20:29(17):2597-605. doi: 10.1089/neu.2012.2348. Epub 2012 Oct 31
[PubMed PMID: 23020190]
[20]
Hang CH, Chen G, Shi JX, Zhang X, Li JS. Cortical expression of nuclear factor kappaB after human brain contusion. Brain research. 2006 Sep 13:1109(1):14-21
[PubMed PMID: 16857176]
[21]
Simard JM, Kilbourne M, Tsymbalyuk O, Tosun C, Caridi J, Ivanova S, Keledjian K, Bochicchio G, Gerzanich V. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. Journal of neurotrauma. 2009 Dec:26(12):2257-67. doi: 10.1089/neu.2009.1021. Epub
[PubMed PMID: 19604096]
[22]
Martínez-Valverde T, Vidal-Jorge M, Martínez-Saez E, Castro L, Arikan F, Cordero E, Rădoi A, Poca MA, Simard JM, Sahuquillo J. Sulfonylurea Receptor 1 in Humans with Post-Traumatic Brain Contusions. Journal of neurotrauma. 2015 Oct 1:32(19):1478-87. doi: 10.1089/neu.2014.3706. Epub 2015 Jun 3
[PubMed PMID: 26398596]
[23]
Gerzanich V, Stokum JA, Ivanova S, Woo SK, Tsymbalyuk O, Sharma A, Akkentli F, Imran Z, Aarabi B, Sahuquillo J, Simard JM. Sulfonylurea Receptor 1, Transient Receptor Potential Cation Channel Subfamily M Member 4, and KIR6.2:Role in Hemorrhagic Progression of Contusion. Journal of neurotrauma. 2019 Apr 1:36(7):1060-1079. doi: 10.1089/neu.2018.5986. Epub 2018 Oct 4
[PubMed PMID: 30160201]
[24]
Jha RM, Bell J, Citerio G, Hemphill JC, Kimberly WT, Narayan RK, Sahuquillo J, Sheth KN, Simard JM. Role of Sulfonylurea Receptor 1 and Glibenclamide in Traumatic Brain Injury: A Review of the Evidence. International journal of molecular sciences. 2020 Jan 9:21(2):. doi: 10.3390/ijms21020409. Epub 2020 Jan 9
[PubMed PMID: 31936452]
[25]
Simard JM, Kahle KT, Gerzanich V. Molecular mechanisms of microvascular failure in central nervous system injury--synergistic roles of NKCC1 and SUR1/TRPM4. Journal of neurosurgery. 2010 Sep:113(3):622-9. doi: 10.3171/2009.11.JNS081052. Epub
[PubMed PMID: 20035575]
[26]
Moscote-Salazar LR, M Rubiano A, Alvis-Miranda HR, Calderon-Miranda W, Alcala-Cerra G, Blancas Rivera MA, Agrawal A. Severe Cranioencephalic Trauma: Prehospital Care, Surgical Management and Multimodal Monitoring. Bulletin of emergency and trauma. 2016 Jan:4(1):8-23
[PubMed PMID: 27162922]
[27]
Ragaisis V. [Brain contusion: morphology, pathogenesis and treatment]. Medicina (Kaunas, Lithuania). 2002:38(3):243-9; quiz 354
[PubMed PMID: 12474694]
[28]
Bouma GJ, Muizelaar JP. Cerebral blood flow in severe clinical head injury. New horizons (Baltimore, Md.). 1995 Aug:3(3):384-94
[PubMed PMID: 7496746]
[29]
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet (London, England). 1974 Jul 13:2(7872):81-4
[PubMed PMID: 4136544]
[30]
Shetty VS, Reis MN, Aulino JM, Berger KL, Broder J, Choudhri AF, Kendi AT, Kessler MM, Kirsch CF, Luttrull MD, Mechtler LL, Prall JA, Raksin PB, Roth CJ, Sharma A, West OC, Wintermark M, Cornelius RS, Bykowski J. ACR Appropriateness Criteria Head Trauma. Journal of the American College of Radiology : JACR. 2016 Jun:13(6):668-79. doi: 10.1016/j.jacr.2016.02.023. Epub
[PubMed PMID: 27262056]
[31]
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, Wilberger J, Surgical Management of Traumatic Brain Injury Author Group. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006 Mar:58(3 Suppl):S25-46; discussion Si-iv
[PubMed PMID: 16540746]
[32]
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Servadei F, Teasdale GM, Pickard JD, Menon DK, Murray GD, Kirkpatrick PJ, RESCUEicp Trial Collaborators. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. The New England journal of medicine. 2016 Sep 22:375(12):1119-30. doi: 10.1056/NEJMoa1605215. Epub 2016 Sep 7
[PubMed PMID: 27602507]
[33]
Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurgical focus. 2009 Jun:26(6):E7. doi: 10.3171/2009.4.FOCUS0965. Epub
[PubMed PMID: 19485720]
[34]
Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017 Jan 1:80(1):6-15. doi: 10.1227/NEU.0000000000001432. Epub
[PubMed PMID: 27654000]
[35]
Hawryluk GWJ, Rubiano AM, Totten AM, O'Reilly C, Ullman JS, Bratton SL, Chesnut R, Harris OA, Kissoon N, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Lumba-Brown A, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury: 2020 Update of the Decompressive Craniectomy Recommendations. Neurosurgery. 2020 Sep 1:87(3):427-434. doi: 10.1093/neuros/nyaa278. Epub
[PubMed PMID: 32761068]
[36]
Nakamura N, Yamaura A, Shigemori M, Ogawa T, Tokutomi T, Ono J, Kawamata T, Sakamoto T. Final report of the Japan Neurotrauma Data Bank project 1998-2001: 1,002 cases of traumatic brain injury. Neurologia medico-chirurgica. 2006 Dec:46(12):567-74
[PubMed PMID: 17185881]
Level 3 (low-level) evidence
[37]
Wang XP, Zhong J, Lei T, Wang HJ, Zhu LN, Chu S, Liu L. Epidemiology of traumatic brain injury-associated epilepsy in western China: An analysis of multicenter data. Epilepsy research. 2020 Aug:164():106354. doi: 10.1016/j.eplepsyres.2020.106354. Epub 2020 May 11
[PubMed PMID: 32438297]
[38]
Brennan PM, Murray GD, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 1: The GCS-Pupils score: an extended index of clinical severity. Journal of neurosurgery. 2018 Jun:128(6):1612-1620. doi: 10.3171/2017.12.JNS172780. Epub 2018 Apr 10
[PubMed PMID: 29631516]
[39]
Murray GD, Brennan PM, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 2: Graphical presentation of probabilities. Journal of neurosurgery. 2018 Jun:128(6):1621-1634. doi: 10.3171/2017.12.JNS172782. Epub 2018 Apr 10
[PubMed PMID: 29631517]
[40]
Jennett B,Bond M, Assessment of outcome after severe brain damage. Lancet (London, England). 1975 Mar 1;
[PubMed PMID: 46957]
[41]
Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, Luerssen TG, Marmarou A, Foulkes MA. The diagnosis of head injury requires a classification based on computed axial tomography. Journal of neurotrauma. 1992 Mar:9 Suppl 1():S287-92
[PubMed PMID: 1588618]
[42]
Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005 Dec:57(6):1173-82; discussion 1173-82
[PubMed PMID: 16331165]
[43]
Huang YH, Deng YH, Lee TC, Chen WF. Rotterdam computed tomography score as a prognosticator in head-injured patients undergoing decompressive craniectomy. Neurosurgery. 2012 Jul:71(1):80-5. doi: 10.1227/NEU.0b013e3182517aa1. Epub
[PubMed PMID: 22382208]
[44]
Charry JD, Falla JD, Ochoa JD, Pinzón MA, Tejada JH, Henriquez MJ, Solano JP, Calvache C. External Validation of the Rotterdam Computed Tomography Score in the Prediction of Mortality in Severe Traumatic Brain Injury. Journal of neurosciences in rural practice. 2017 Aug:8(Suppl 1):S23-S26. doi: 10.4103/jnrp.jnrp_434_16. Epub
[PubMed PMID: 28936067]
Level 1 (high-level) evidence