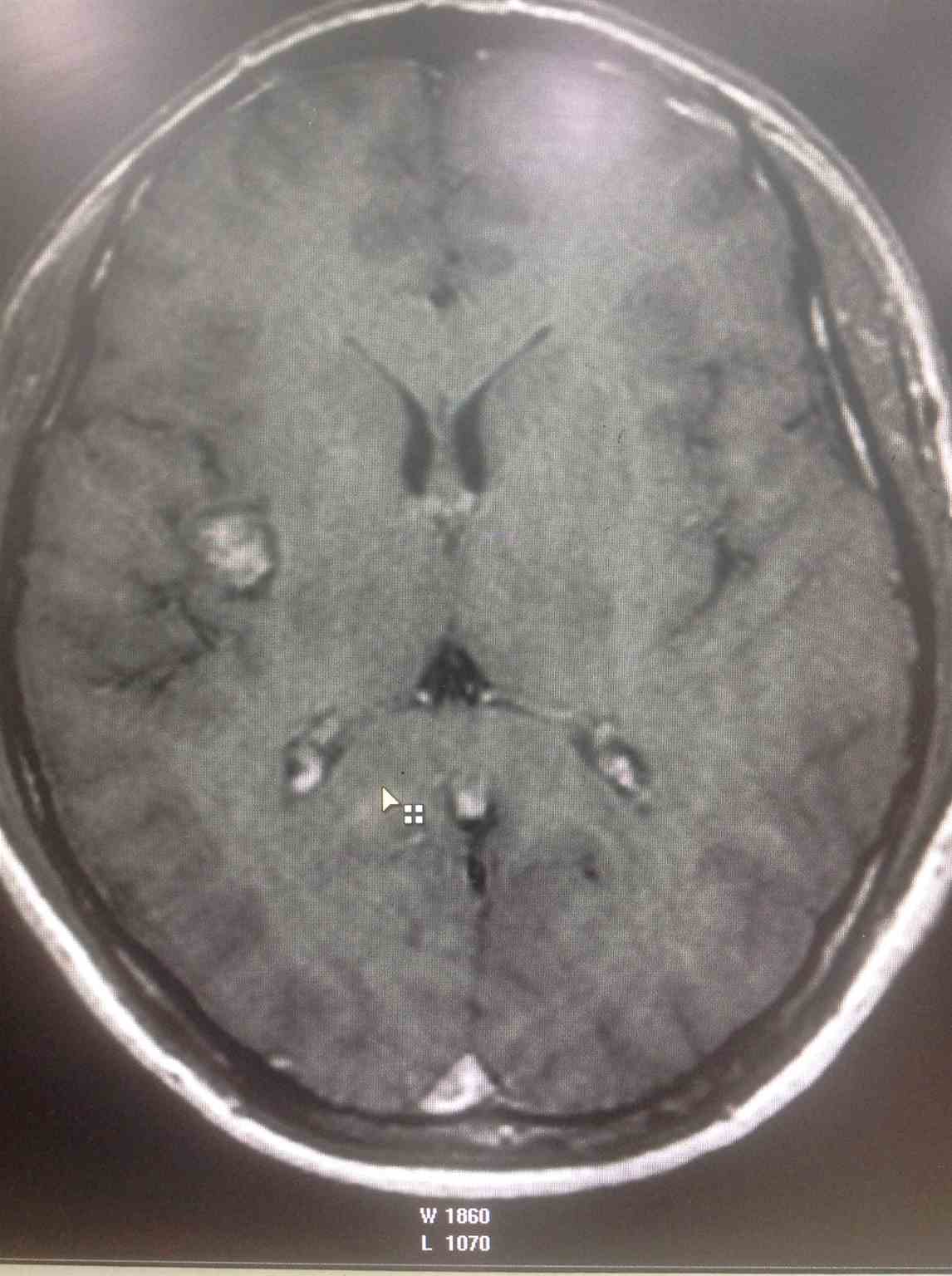
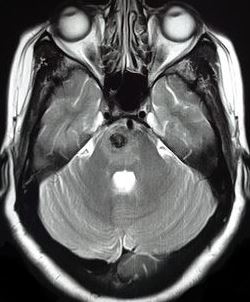
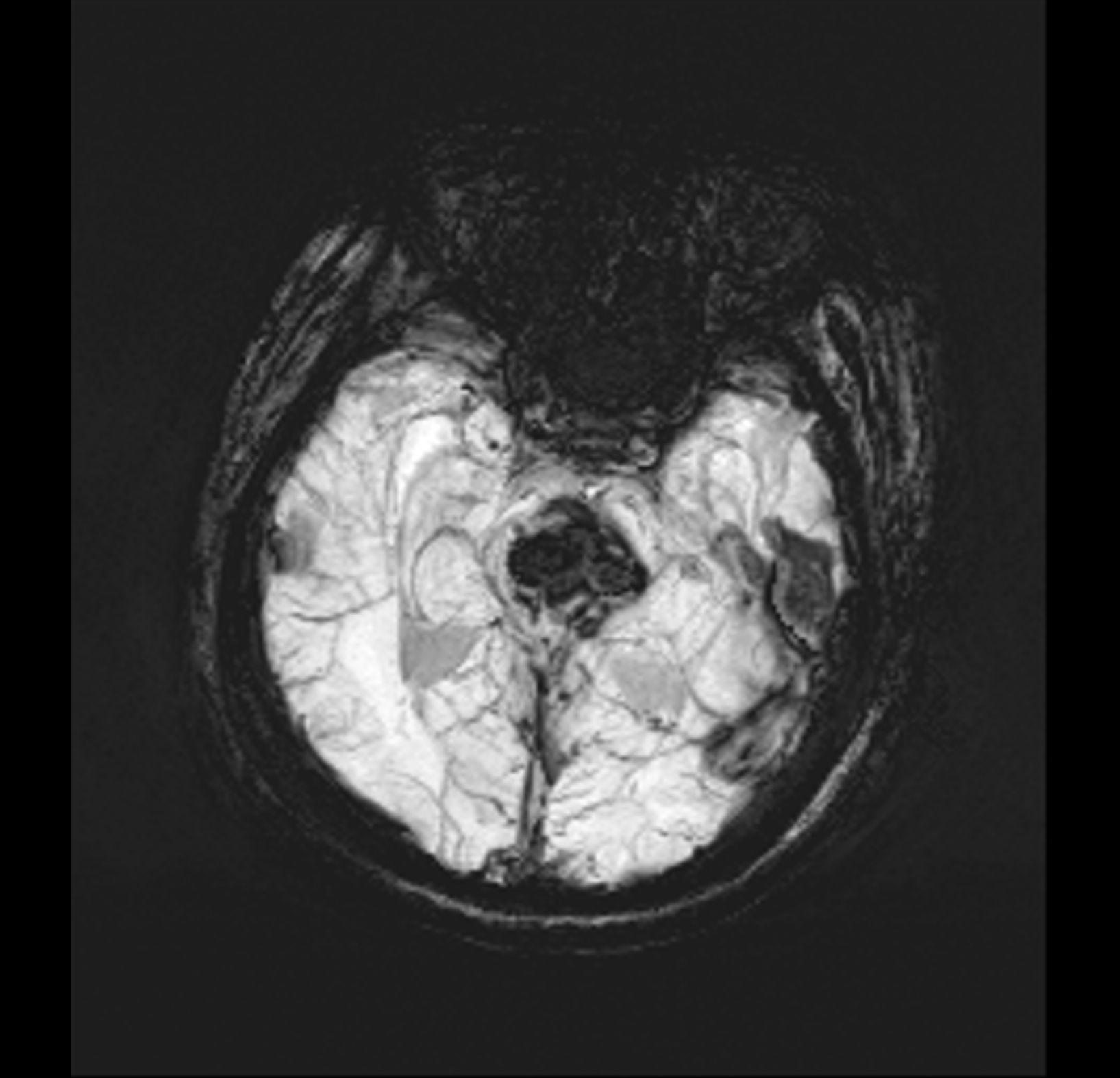
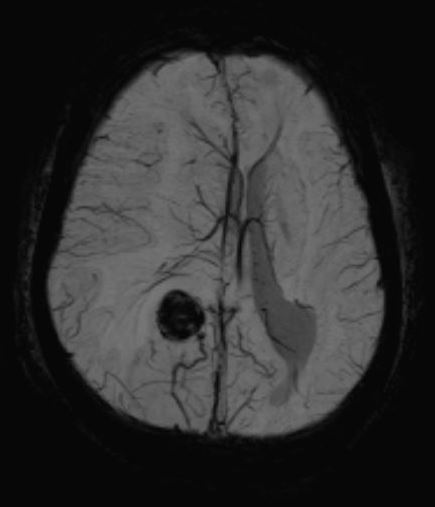
[1]
Khattar NK, Adams SW, Schaber AS, White AC, Al Ghamdi M, Hruska RT, Savage JJ, Downs RK, Hattab EM, Williams BJ. Endoscopic Endonasal Surgery for the Resection of a Cavernous Hemangioma with a Sellar Extension. Cureus. 2018 Nov 30:10(11):e3663. doi: 10.7759/cureus.3663. Epub 2018 Nov 30
[PubMed PMID: 30740283]
[2]
Li H, Zhang B, Wang W, Wei MH, Liu BY, Wu Z. Clinical Features, Intradural Transcavernous Surgical Management, and Outcomes of Giant Cavernous Sinus Hemangiomas: A Single-Institution Experience. World neurosurgery. 2019 May:125():e754-e763. doi: 10.1016/j.wneu.2019.01.165. Epub 2019 Feb 5
[PubMed PMID: 30735865]
[3]
Pandey SK, Mani SE, Sudhakar SV, Panwar J, Joseph BV, Rajshekhar V. Reliability of Imaging-Based Diagnosis of Lateral Ventricular Masses in Children. World neurosurgery. 2019 Apr:124():e693-e701. doi: 10.1016/j.wneu.2018.12.196. Epub 2019 Jan 17
[PubMed PMID: 30660880]
[4]
Li W, Sun Q, Duan X, Yi F, Zhou Y, Hu Y, Yao L, Xu H, Zhou L. [Etiologies and risk factors for young people with intracerebral hemorrhage]. Zhong nan da xue xue bao. Yi xue ban = Journal of Central South University. Medical sciences. 2018 Nov 28:43(11):1246-1250. doi: 10.11817/j.issn.1672-7347.2018.11.013. Epub
[PubMed PMID: 30643071]
[5]
Villalonga JF,Saenz A,Campero A, Surgical treatment of an asymptomatic giant supratentorial cavernous hemangioma. Case report. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2019 Jan 4;
[PubMed PMID: 30616875]
Level 3 (low-level) evidence
[6]
Apra C, Dumot C, Bourdillon P, Pelissou-Guyotat I. Could propranolol be beneficial in adult cerebral cavernous malformations? Neurosurgical review. 2019 Jun:42(2):403-408. doi: 10.1007/s10143-018-01074-0. Epub 2019 Jan 4
[PubMed PMID: 30610500]
[7]
Li ZH, Wu Z, Zhang JT, Zhang LW. Surgical Management and Outcomes of Cavernous Sinus Hemangiomas: A Single-Institution Series of 47 Patients. World neurosurgery. 2019 Feb:122():e1181-e1194. doi: 10.1016/j.wneu.2018.11.015. Epub 2018 Nov 14
[PubMed PMID: 30447442]
[8]
Pesce A, Frati A, D'Andrea G, Palmieri M, Familiari P, Cimatti M, Valente D, Raco A. The Real Impact of an Intraoperative Magnetic Resonance Imaging-Equipped Operative Theatre in Neurovascular Surgery: The Sapienza University Experience. World neurosurgery. 2018 Dec:120():190-199. doi: 10.1016/j.wneu.2018.08.124. Epub 2018 Aug 27
[PubMed PMID: 30165208]
[9]
Zacà D, Corsini F, Rozzanigo U, Dallabona M, Avesani P, Annicchiarico L, Zigiotto L, Faraca G, Chioffi F, Jovicich J, Sarubbo S. Whole-Brain Network Connectivity Underlying the Human Speech Articulation as Emerged Integrating Direct Electric Stimulation, Resting State fMRI and Tractography. Frontiers in human neuroscience. 2018:12():405. doi: 10.3389/fnhum.2018.00405. Epub 2018 Oct 11
[PubMed PMID: 30364298]
[10]
Tang X,Li M,Xu Y,Xu M,Sun W,Li G,Xu N,Wang L, TMS-induced motor evoked potential in the preliminary diagnosis of intracranial cavernous hemangioma: A case report. Brain stimulation. 2018 Nov - Dec;
[PubMed PMID: 30087033]
Level 3 (low-level) evidence
[11]
Lee CC, Chou CL, Chen CJ, Yang HC, Wu HM, Shiau CY, Pan DH, Chung WY. Stereotactic radiosurgery for hypervascular intracranial tumors. Journal of neuro-oncology. 2018 Dec:140(3):547-558. doi: 10.1007/s11060-018-2980-8. Epub 2018 Aug 20
[PubMed PMID: 30128688]
[12]
Xie MG, Xiao XR, Guo FZ, Zhang JT, Wu Z, Zhang LW. Surgical Management and Functional Outcomes of Cavernous Malformations Involving the Medulla Oblongata. World neurosurgery. 2018 Nov:119():e643-e652. doi: 10.1016/j.wneu.2018.07.229. Epub 2018 Aug 2
[PubMed PMID: 30077748]
[13]
Barbagallo GMV, Morrone A, Certo F. Intraoperative Computed Tomography and Awake Craniotomy: A Useful and Safe Combination in Brain Surgery. World neurosurgery. 2018 Nov:119():e159-e166. doi: 10.1016/j.wneu.2018.07.078. Epub 2018 Jul 18
[PubMed PMID: 30031198]
[14]
Xie MG, Xiao XR, Li D, Guo FZ, Zhang JT, Wu Z, Zhang LW. Surgical Treatment of the Medullary Cavernous Malformations: 53 Cases. World neurosurgery. 2018 Oct:118():e449-e459. doi: 10.1016/j.wneu.2018.06.213. Epub 2018 Jul 4
[PubMed PMID: 29981463]
Level 3 (low-level) evidence