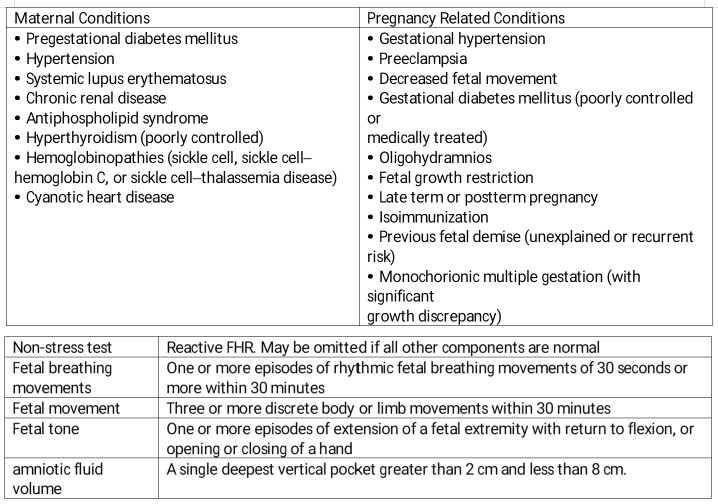
Introduction
Point of care ultrasound (POCUS) in obstetrics is an invaluable tool in the evaluation of the pregnant patient. Given its ease of use and interpretation, a quick assessment can provide important information regarding the management of obstetric concerns. The biophysical profile (BPP) is a maximum 30-minute long ultrasound assessment to assess fetal well-being coupled with a fetal heart rate tracing. The elements are a non-stress test, assessment of fluid index, fetal breathing movements, total body movements, and limb tone demonstrated by flexion and extension of the limbs. The modified BPP is a shortened study that involves a non-stress test (NST) and amniotic fluid index (AFI). See Image. Ultrasound Biophysical Profile Table.
The history of this test has been the source of investigation from the 1970s to the 1990s. Initial studies were in fetal lambs and analyzed the cardiovascular and metabolic changes experienced in fetal lambs under controlled environments that altered the maternal pCO2 and cardiac output. Reflections in the fetal-placental, umbilical, and cardiovascular hemodynamics were monitored using vascular catheters and blood sampling. Additionally, the ultrasound findings and fetal heart rate (FHR) patterns were studied. In the 1990s, the correlation between these variables came together in a landmark study that identified a linear relationship between the BPP score and umbilical cord venous pH sampled via cordocentesis. Scores of 8 and 10 consistently had normal cord pH levels. However, a score of 0 exhibited a mean cord pH of 7.07 prompting urgent intervention or delivery. Researchers were able to break down the scores further and found that abnormal amniotic fluid correlated with a lower pH compared to short term abnormalities such as an abnormal NST or fetal breathing movements.[1] This ultimately provides the rationale for BPP scoring and obstetric management. The modified BPP provides almost as powerful a study as the full BPP and is normally part of antenatal testing for high risk maternal and fetal conditions.