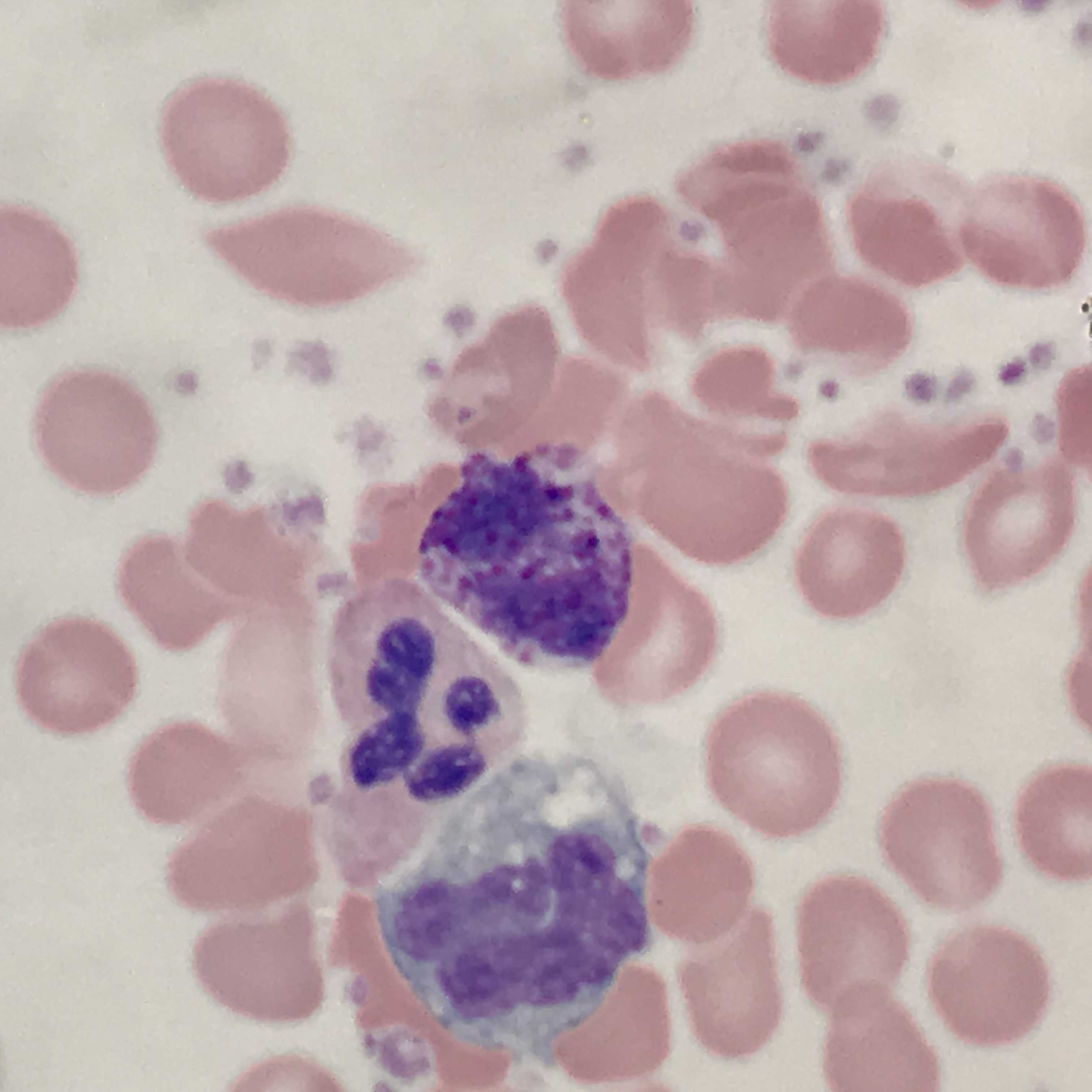
[1]
Valent P, Sotlar K, Blatt K, Hartmann K, Reiter A, Sadovnik I, Sperr WR, Bettelheim P, Akin C, Bauer K, George TI, Hadzijusufovic E, Wolf D, Gotlib J, Mahon FX, Metcalfe DD, Horny HP, Arock M. Proposed diagnostic criteria and classification of basophilic leukemias and related disorders. Leukemia. 2017 Apr:31(4):788-797. doi: 10.1038/leu.2017.15. Epub 2017 Jan 16
[PubMed PMID: 28090091]
[2]
Boiten HJ, de Jongh E. Atypical basophilia. Blood. 2018 Aug 2:132(5):551. doi: 10.1182/blood-2018-05-849901. Epub
[PubMed PMID: 30072416]
[3]
Han X, Jorgensen JL, Brahmandam A, Schlette E, Huh YO, Shi Y, Awagu S, Chen W. Immunophenotypic study of basophils by multiparameter flow cytometry. Archives of pathology & laboratory medicine. 2008 May:132(5):813-9
[PubMed PMID: 18466030]
[4]
Sadovnik I, Ivanov D, Smiljkovic D, Stefanzl G, Degenfeld-Schonburg L, Herndlhofer S, Eisenwort G, Hauswirth AW, Sliwa T, Keil F, Sperr WR, Valent P. Identification of CD203c as a New Basophil-Specific Flow-Marker in Ph(+) Chronic Myeloid Leukemia. Cells. 2022 Dec 20:12(1):. doi: 10.3390/cells12010003. Epub 2022 Dec 20
[PubMed PMID: 36611797]
[5]
Arinobu Y, Iwasaki H, Akashi K. Origin of basophils and mast cells. Allergology international : official journal of the Japanese Society of Allergology. 2009 Mar:58(1):21-8. doi: 10.2332/allergolint.08-RAI-0067. Epub 2009 Jan 25
[PubMed PMID: 19153533]
[6]
Arock M, Schneider E, Boissan M, Tricottet V, Dy M. Differentiation of human basophils: an overview of recent advances and pending questions. Journal of leukocyte biology. 2002 Apr:71(4):557-64
[PubMed PMID: 11927641]
Level 3 (low-level) evidence
[7]
Min B, Brown MA, Legros G. Understanding the roles of basophils: breaking dawn. Immunology. 2012 Mar:135(3):192-7. doi: 10.1111/j.1365-2567.2011.03530.x. Epub
[PubMed PMID: 22044049]
Level 3 (low-level) evidence
[8]
Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. The Journal of allergy and clinical immunology. 2010 Feb:125(2 Suppl 2):S73-80. doi: 10.1016/j.jaci.2009.11.017. Epub
[PubMed PMID: 20176269]
[9]
Korošec P, Gibbs BF, Rijavec M, Custovic A, Turner PJ. Important and specific role for basophils in acute allergic reactions. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2018 May:48(5):502-512. doi: 10.1111/cea.13117. Epub 2018 Mar 23
[PubMed PMID: 29431885]
[10]
Siracusa MC, Kim BS, Spergel JM, Artis D. Basophils and allergic inflammation. The Journal of allergy and clinical immunology. 2013 Oct:132(4):789-801; quiz 788. doi: 10.1016/j.jaci.2013.07.046. Epub
[PubMed PMID: 24075190]
[11]
Tanaka Y, Tanaka A, Hashimoto A, Hayashi K, Shinzato I. Acute Myeloid Leukemia with Basophilic Differentiation Transformed from Myelodysplastic Syndrome. Case reports in hematology. 2017:2017():4695491. doi: 10.1155/2017/4695491. Epub 2017 Mar 27
[PubMed PMID: 28428897]
Level 3 (low-level) evidence
[14]
Chirumbolo S. State-of-the-art review about basophil research in immunology and allergy: is the time right to treat these cells with the respect they deserve? Blood transfusion = Trasfusione del sangue. 2012 Apr:10(2):148-64. doi: 10.2450/2011.0020-11. Epub 2011 Dec 21
[PubMed PMID: 22244003]
[15]
Kovalszki A, Weller PF. Eosinophilia. Primary care. 2016 Dec:43(4):607-617. doi: 10.1016/j.pop.2016.07.010. Epub 2016 Oct 14
[PubMed PMID: 27866580]
[16]
Chhabra G. Automated hematology analyzers: Recent trends and applications. Journal of laboratory physicians. 2018 Jan-Mar:10(1):15-16. doi: 10.4103/JLP.JLP_124_17. Epub
[PubMed PMID: 29403197]
[17]
Arneth BM, Menschikowki M. Technology and new fluorescence flow cytometry parameters in hematological analyzers. Journal of clinical laboratory analysis. 2015 May:29(3):175-83. doi: 10.1002/jcla.21747. Epub 2014 May 5
[PubMed PMID: 24797912]
[18]
Sireci A, Schlaberg R, Kratz A. A method for optimizing and validating institution-specific flagging criteria for automated cell counters. Archives of pathology & laboratory medicine. 2010 Oct:134(10):1528-33
[PubMed PMID: 20923310]
[19]
Michael HT, Nabity MB, Couto CG, Moritz A, Harvey JW, DeNicola DB, Hammond JM. Improving quality control for in-clinic hematology analyzers: Common myths and opportunities. Veterinary clinical pathology. 2022 Sep:51(3):302-310. doi: 10.1111/vcp.13154. Epub
[PubMed PMID: 36097323]
Level 2 (mid-level) evidence
[20]
Giannoli JM, Albarede S, Avellan T, Bouilloux JP, Cartier R, Cohen R, Colard N, Essemilaire L, Galinier JL, Kuentz M, Paris M, Portugal H, Scherrer F, Siest JP, Vassault A, Vialle JM. Recommendations for the application and follow-up of quality controls in medical laboratories. Biochemia medica. 2021 Jun 15:31(2):020501. doi: 10.11613/BM.2021.020501. Epub 2021 Apr 15
[PubMed PMID: 33927549]
Level 2 (mid-level) evidence
[21]
Gulati G, Song J, Florea AD, Gong J. Purpose and criteria for blood smear scan, blood smear examination, and blood smear review. Annals of laboratory medicine. 2013 Jan:33(1):1-7. doi: 10.3343/alm.2013.33.1.1. Epub 2012 Dec 17
[PubMed PMID: 23301216]
[22]
Thompson PA, Kantarjian HM, Cortes JE. Diagnosis and Treatment of Chronic Myeloid Leukemia in 2015. Mayo Clinic proceedings. 2015 Oct:90(10):1440-54. doi: 10.1016/j.mayocp.2015.08.010. Epub
[PubMed PMID: 26434969]
[23]
May PC, Reid AG, Robinson ME, Khorashad JS, Milojkovic D, Claudiani S, Genomics England Research Consortium, Willis F, Apperley JF, Innes AJ. FISH-negative BCR::ABL1-positive e19a2 chronic myeloid leukaemia: the most cryptic of insertions. BMC medical genomics. 2023 Jul 26:16(1):172. doi: 10.1186/s12920-023-01607-7. Epub 2023 Jul 26
[PubMed PMID: 37496024]
[24]
Viny AD, Levine RL. Genetics of myeloproliferative neoplasms. Cancer journal (Sudbury, Mass.). 2014 Jan-Feb:20(1):61-5. doi: 10.1097/PPO.0000000000000013. Epub
[PubMed PMID: 24445766]
[25]
Valent P, Horny HP, Arock M. The underestimated role of basophils in Ph(+) chronic myeloid leukaemia. European journal of clinical investigation. 2018 Oct:48(10):e13000. doi: 10.1111/eci.13000. Epub 2018 Aug 6
[PubMed PMID: 30019447]
[26]
Zhou J, Papenhausen P, Shao H. Therapy-related acute myeloid leukemia with eosinophilia, basophilia, t(4;14)(q12;q24) and PDGFRA rearrangement: a case report and review of the literature. International journal of clinical and experimental pathology. 2015:8(5):5812-20
[PubMed PMID: 26191303]
Level 3 (low-level) evidence
[28]
Stock W, Hoffman R. White blood cells 1: non-malignant disorders. Lancet (London, England). 2000 Apr 15:355(9212):1351-7
[PubMed PMID: 10776761]
[29]
Pastoret C, Houot R. "Chronic myelogenous leukemia in primary blast crisis" rather than "de novo BCR-ABL1-positive acute myeloid leukemia". Clinical case reports. 2017 Jun:5(6):757-760. doi: 10.1002/ccr3.937. Epub 2017 Apr 4
[PubMed PMID: 28588805]
Level 3 (low-level) evidence