[1]
Latgé JP, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clinical microbiology reviews. 2019 Dec 18:33(1):. doi: 10.1128/CMR.00140-18. Epub 2019 Nov 13
[PubMed PMID: 31722890]
[2]
Jenks JD, Hoenigl M. Treatment of Aspergillosis. Journal of fungi (Basel, Switzerland). 2018 Aug 19:4(3):. doi: 10.3390/jof4030098. Epub 2018 Aug 19
[PubMed PMID: 30126229]
[3]
Henß I, Kleinemeier C, Strobel L, Brock M, Löffler J, Ebel F. Characterization of Aspergillus terreus Accessory Conidia and Their Interactions With Murine Macrophages. Frontiers in microbiology. 2022:13():896145. doi: 10.3389/fmicb.2022.896145. Epub 2022 Jun 16
[PubMed PMID: 35783442]
[4]
Gibbons JG, Rokas A. The function and evolution of the Aspergillus genome. Trends in microbiology. 2013 Jan:21(1):14-22. doi: 10.1016/j.tim.2012.09.005. Epub 2012 Oct 17
[PubMed PMID: 23084572]
[5]
Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respiratory medicine. 2018 Aug:141():121-131. doi: 10.1016/j.rmed.2018.06.029. Epub 2018 Jul 3
[PubMed PMID: 30053957]
[6]
Montone KT. Pathology of Fungal Rhinosinusitis: A Review. Head and neck pathology. 2016 Mar:10(1):40-46. doi: 10.1007/s12105-016-0690-0. Epub 2016 Feb 1
[PubMed PMID: 26830404]
[7]
Thompson GR 3rd, Young JH. Aspergillus Infections. The New England journal of medicine. 2021 Oct 14:385(16):1496-1509. doi: 10.1056/NEJMra2027424. Epub
[PubMed PMID: 34644473]
[8]
Denning DW. Aspergillosis: diagnosis and treatment. International journal of antimicrobial agents. 1996 Feb:6(3):161-8
[PubMed PMID: 18611704]
[9]
Lamoth F, Calandra T. Let's add invasive aspergillosis to the list of influenza complications. The Lancet. Respiratory medicine. 2018 Oct:6(10):733-735. doi: 10.1016/S2213-2600(18)30332-1. Epub 2018 Jul 31
[PubMed PMID: 30076120]
[10]
Hoenigl M, Gangneux JP, Segal E, Alanio A, Chakrabarti A, Chen SC, Govender N, Hagen F, Klimko N, Meis JF, Pasqualotto AC, Seidel D, Walsh TJ, Lagrou K, Lass-Flörl C, Cornely OA, European Confederation of Medical Mycology (ECMM). Global guidelines and initiatives from the European Confederation of Medical Mycology to improve patient care and research worldwide: New leadership is about working together. Mycoses. 2018 Nov:61(11):885-894. doi: 10.1111/myc.12836. Epub 2018 Aug 28
[PubMed PMID: 30086186]
[11]
Wéry N. Bioaerosols from composting facilities--a review. Frontiers in cellular and infection microbiology. 2014:4():42. doi: 10.3389/fcimb.2014.00042. Epub 2014 Apr 4
[PubMed PMID: 24772393]
[12]
Chakrabarti A, Chatterjee SS, Das A, Shivaprakash MR. Invasive aspergillosis in developing countries. Medical mycology. 2011 Apr:49 Suppl 1():S35-47. doi: 10.3109/13693786.2010.505206. Epub 2010 Aug 18
[PubMed PMID: 20718613]
[13]
Smith TC, Benefield RJ, Kim JH. Risk of Fungal Endophthalmitis Associated with Cataract Surgery: A Mini-Review. Mycopathologia. 2015 Dec:180(5-6):291-7. doi: 10.1007/s11046-015-9932-z. Epub 2015 Aug 29
[PubMed PMID: 26318595]
[14]
Sionov E, Sandovsky-Losica H, Gov Y, Segal E. Adherence of Aspergillus species to soft contact lenses and attempts to inhibit the adherence. Mycoses. 2001 Dec:44(11-12):464-71
[PubMed PMID: 11820259]
[15]
Manikandan P, Abdel-Hadi A, Randhir Babu Singh Y, Revathi R, Anita R, Banawas S, Bin Dukhyil AA, Alshehri B, Shobana CS, Panneer Selvam K, Narendran V. Fungal Keratitis: Epidemiology, Rapid Detection, and Antifungal Susceptibilities of Fusarium and Aspergillus Isolates from Corneal Scrapings. BioMed research international. 2019:2019():6395840. doi: 10.1155/2019/6395840. Epub 2019 Jan 20
[PubMed PMID: 30800674]
[16]
Koutserimpas C, Chamakioti I, Raptis K, Alpantaki K, Vrioni G, Samonis G. Osseous Infections Caused by Aspergillus Species. Diagnostics (Basel, Switzerland). 2022 Jan 14:12(1):. doi: 10.3390/diagnostics12010201. Epub 2022 Jan 14
[PubMed PMID: 35054368]
[17]
Routray C, Nwaigwe C. Sternal osteomyelitis secondary to Aspergillus fumigatus after cardiothoracic surgery. Medical mycology case reports. 2020 Jun:28():16-19. doi: 10.1016/j.mmcr.2020.03.003. Epub 2020 Mar 25
[PubMed PMID: 32274324]
Level 3 (low-level) evidence
[18]
Darr-Foit S, Schliemann S, Scholl S, Hipler UC, Elsner P. Primary cutaneous aspergillosis - an uncommon opportunistic infection Review of the literature and case presentation. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2017 Aug:15(8):839-841. doi: 10.1111/ddg.13297. Epub
[PubMed PMID: 28763605]
Level 3 (low-level) evidence
[19]
Norbury W, Herndon DN, Tanksley J, Jeschke MG, Finnerty CC. Infection in Burns. Surgical infections. 2016 Apr:17(2):250-5. doi: 10.1089/sur.2013.134. Epub
[PubMed PMID: 26978531]
[20]
Bongomin F, Batac CR, Richardson MD, Denning DW. A Review of Onychomycosis Due to Aspergillus Species. Mycopathologia. 2018 Jun:183(3):485-493. doi: 10.1007/s11046-017-0222-9. Epub 2017 Nov 16
[PubMed PMID: 29147866]
[21]
Strickland AB, Shi M. Mechanisms of fungal dissemination. Cellular and molecular life sciences : CMLS. 2021 Apr:78(7):3219-3238. doi: 10.1007/s00018-020-03736-z. Epub 2021 Jan 15
[PubMed PMID: 33449153]
[22]
Valerio M, Camici M, Machado M, Galar A, Olmedo M, Sousa D, Antorrena-Miranda I, Fariñas MC, Hidalgo-Tenorio C, Montejo M, Vena A, Guinea J, Bouza E, Muñoz P, GAMES Study Group. Aspergillus endocarditis in the recent years, report of cases of a multicentric national cohort and literature review. Mycoses. 2022 Mar:65(3):362-373. doi: 10.1111/myc.13415. Epub 2022 Jan 12
[PubMed PMID: 34931375]
Level 3 (low-level) evidence
[23]
Ceylan B, Yilmaz M, Beköz HS, Ramadan S, Ertan Akan G, Mert A. Primary gastrointestinal aspergillosis: a case report and literature review. Le infezioni in medicina. 2019 Mar 1:27(1):85-92
[PubMed PMID: 30882385]
Level 3 (low-level) evidence
[24]
Kidd SE, Chen SC, Meyer W, Halliday CL. A New Age in Molecular Diagnostics for Invasive Fungal Disease: Are We Ready? Frontiers in microbiology. 2019:10():2903. doi: 10.3389/fmicb.2019.02903. Epub 2020 Jan 14
[PubMed PMID: 31993022]
[25]
Abad A, Fernández-Molina JV, Bikandi J, Ramírez A, Margareto J, Sendino J, Hernando FL, Pontón J, Garaizar J, Rementeria A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Revista iberoamericana de micologia. 2010 Oct-Dec:27(4):155-82. doi: 10.1016/j.riam.2010.10.003. Epub 2010 Oct 23
[PubMed PMID: 20974273]
[26]
Wang Y, Zhang L, Zhou L, Zhang M, Xu Y. Epidemiology, Drug Susceptibility, and Clinical Risk Factors in Patients With Invasive Aspergillosis. Frontiers in public health. 2022:10():835092. doi: 10.3389/fpubh.2022.835092. Epub 2022 Apr 15
[PubMed PMID: 35493371]
[27]
Khoo SH, Denning DW. Invasive aspergillosis in patients with AIDS. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1994 Aug:19 Suppl 1():S41-8
[PubMed PMID: 7948570]
[28]
van de Peppel RJ, Visser LG, Dekkers OM, de Boer MGJ. The burden of Invasive Aspergillosis in patients with haematological malignancy: A meta-analysis and systematic review. The Journal of infection. 2018 Jun:76(6):550-562. doi: 10.1016/j.jinf.2018.02.012. Epub 2018 May 1
[PubMed PMID: 29727605]
Level 1 (high-level) evidence
[29]
Abers MS, Ghebremichael MS, Timmons AK, Warren HS, Poznansky MC, Vyas JM. A Critical Reappraisal of Prolonged Neutropenia as a Risk Factor for Invasive Pulmonary Aspergillosis. Open forum infectious diseases. 2016 Jan:3(1):ofw036. doi: 10.1093/ofid/ofw036. Epub 2016 Feb 12
[PubMed PMID: 27006961]
[30]
Bassetti M, Bouza E. Invasive mould infections in the ICU setting: complexities and solutions. The Journal of antimicrobial chemotherapy. 2017 Mar 1:72(suppl_1):i39-i47. doi: 10.1093/jac/dkx032. Epub
[PubMed PMID: 28355466]
[31]
Morgan J, Wannemuehler KA, Marr KA, Hadley S, Kontoyiannis DP, Walsh TJ, Fridkin SK, Pappas PG, Warnock DW. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Medical mycology. 2005 May:43 Suppl 1():S49-58
[PubMed PMID: 16110792]
[32]
Jenks JD, Nam HH, Hoenigl M. Invasive aspergillosis in critically ill patients: Review of definitions and diagnostic approaches. Mycoses. 2021 Sep:64(9):1002-1014. doi: 10.1111/myc.13274. Epub 2021 Apr 6
[PubMed PMID: 33760284]
[33]
Robinson KM. Mechanistic Basis of Super-Infection: Influenza-Associated Invasive Pulmonary Aspergillosis. Journal of fungi (Basel, Switzerland). 2022 Apr 22:8(5):. doi: 10.3390/jof8050428. Epub 2022 Apr 22
[PubMed PMID: 35628684]
[34]
Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, Fornaro G, Tonetti T, Pizzilli G, Francalanci E, Giuntoli L, Rubin A, Moroni A, Ambretti S, Trapani F, Vatamanu O, Ranieri VM, Castelli A, Baiocchi M, Lewis R, Giannella M, Viale P, PREDICO Study Group. Epidemiology of Invasive Pulmonary Aspergillosis Among Intubated Patients With COVID-19: A Prospective Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021 Dec 6:73(11):e3606-e3614. doi: 10.1093/cid/ciaa1065. Epub
[PubMed PMID: 32719848]
[35]
Zilberberg MD, Harrington R, Spalding JR, Shorr AF. Burden of hospitalizations over time with invasive aspergillosis in the United States, 2004-2013. BMC public health. 2019 May 17:19(1):591. doi: 10.1186/s12889-019-6932-9. Epub 2019 May 17
[PubMed PMID: 31101036]
[36]
Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, Elhaddad A, Frutos C, Galeano S, Hamad N, Hamidieh AA, Hashmi S, Ho A, Horowitz MM, Iida M, Jaimovich G, Karduss A, Kodera Y, Kröger N, Péffault de Latour R, Lee JW, Martínez-Rolón J, Pasquini MC, Passweg J, Paulson K, Seber A, Snowden JA, Srivastava A, Szer J, Weisdorf D, Worel N, Koh MBC, Aljurf M, Greinix H, Atsuta Y, Saber W. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. 2022 May 1:107(5):1045-1053. doi: 10.3324/haematol.2021.279189. Epub 2022 May 1
[PubMed PMID: 34382386]
[37]
Agarwal R, Sehgal IS, Dhooria S, Muthu V, Prasad KT, Bal A, Aggarwal AN, Chakrabarti A. Allergic bronchopulmonary aspergillosis. The Indian journal of medical research. 2020 Jun:151(6):529-549. doi: 10.4103/ijmr.IJMR_1187_19. Epub
[PubMed PMID: 32719226]
[38]
Sabino R, Veríssimo C, Viegas C, Viegas S, Brandão J, Alves-Correia M, Borrego LM, Clemons KV, Stevens DA, Richardson M. The role of occupational Aspergillus exposure in the development of diseases. Medical mycology. 2019 Apr 1:57(Supplement_2):S196-S205. doi: 10.1093/mmy/myy090. Epub
[PubMed PMID: 30816970]
[39]
Benedict K, Thompson GR 3rd, Jackson BR. Cannabis Use and Fungal Infections in a Commercially Insured Population, United States, 2016. Emerging infectious diseases. 2020 Jun:26(6):1308-1310. doi: 10.3201/eid2606.191570. Epub
[PubMed PMID: 32441624]
[40]
Zilberberg MD, Nathanson BH, Harrington R, Spalding JR, Shorr AF. Epidemiology and Outcomes of Hospitalizations With Invasive Aspergillosis in the United States, 2009-2013. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018 Aug 16:67(5):727-735. doi: 10.1093/cid/ciy181. Epub
[PubMed PMID: 29718296]
[41]
Feldmesser M. Role of neutrophils in invasive aspergillosis. Infection and immunity. 2006 Dec:74(12):6514-6
[PubMed PMID: 17030575]
[42]
Alanio A, Bretagne S. Challenges in microbiological diagnosis of invasive Aspergillus infections. F1000Research. 2017:6():. pii: F1000 Faculty Rev-157. doi: 10.12688/f1000research.10216.1. Epub 2017 Feb 17
[PubMed PMID: 28299183]
[43]
Lass-Flörl C. How to make a fast diagnosis in invasive aspergillosis. Medical mycology. 2019 Apr 1:57(Supplement_2):S155-S160. doi: 10.1093/mmy/myy103. Epub
[PubMed PMID: 30816965]
[44]
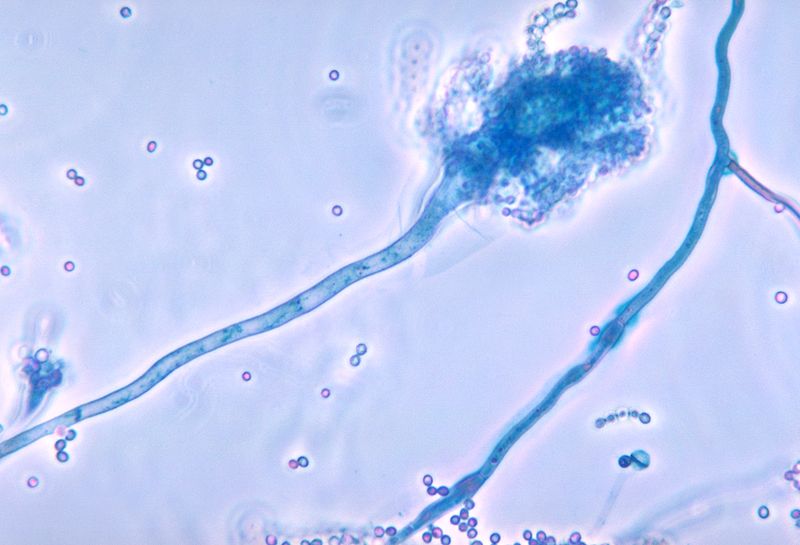
Sugui JA, Kwon-Chung KJ, Juvvadi PR, Latgé JP, Steinbach WJ. Aspergillus fumigatus and related species. Cold Spring Harbor perspectives in medicine. 2014 Nov 6:5(2):a019786. doi: 10.1101/cshperspect.a019786. Epub 2014 Nov 6
[PubMed PMID: 25377144]
Level 3 (low-level) evidence
[45]
Fernández-Cruz A, Magira E, Heo ST, Evans S, Tarrand J, Kontoyiannis DP. Bronchoalveolar Lavage Fluid Cytology in Culture-Documented Invasive Pulmonary Aspergillosis in Patients with Hematologic Diseases: Analysis of 67 Episodes. Journal of clinical microbiology. 2018 Oct:56(10):. doi: 10.1128/JCM.00962-18. Epub 2018 Sep 25
[PubMed PMID: 30021823]
[46]
Gletsou E, Ioannou M, Liakopoulos V, Tsiambas E, Ragos V, Stefanidis I. Aspergillosis in immunocompromised patients with haematological malignancies. Journal of B.U.ON. : official journal of the Balkan Union of Oncology. 2018 Dec:23(7):7-10
[PubMed PMID: 30722105]
[47]
Fung M, Babik J, Humphreys IM, Davis GE. Diagnosis and Treatment of Acute Invasive Fungal Sinusitis in Cancer and Transplant Patients. Current infectious disease reports. 2019 Nov 26:21(12):53. doi: 10.1007/s11908-019-0707-4. Epub 2019 Nov 26
[PubMed PMID: 31773398]
[48]
Howells RC, Ramadan HH. Usefulness of computed tomography and magnetic resonance in fulminant invasive fungal rhinosinusitis. American journal of rhinology. 2001 Jul-Aug:15(4):255-61
[PubMed PMID: 11554658]
[49]
Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Aug 15:63(4):e1-e60. doi: 10.1093/cid/ciw326. Epub 2016 Jun 29
[PubMed PMID: 27365388]
Level 1 (high-level) evidence
[50]
Samanta P, Clancy CJ, Nguyen MH. Fungal infections in lung transplantation. Journal of thoracic disease. 2021 Nov:13(11):6695-6707. doi: 10.21037/jtd-2021-26. Epub
[PubMed PMID: 34992845]
[51]
Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Flörl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M, Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Brüggemann RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A, Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux JP, Garbino J, Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ, Lange C, Lehrnbecher T, Löffler J, Lortholary O, Maertens J, Marchetti O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M, Sanguinetti M, Sheppard DC, Sinkó J, Skiada A, Vehreschild MJGT, Viscoli C, Cornely OA. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2018 May:24 Suppl 1():e1-e38. doi: 10.1016/j.cmi.2018.01.002. Epub 2018 Mar 12
[PubMed PMID: 29544767]
[52]
Maertens JA, Klont R, Masson C, Theunissen K, Meersseman W, Lagrou K, Heinen C, Crépin B, Van Eldere J, Tabouret M, Donnelly JP, Verweij PE. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007 May 15:44(10):1329-36
[PubMed PMID: 17443470]
[53]
D'Haese J, Theunissen K, Vermeulen E, Schoemans H, De Vlieger G, Lammertijn L, Meersseman P, Meersseman W, Lagrou K, Maertens J. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: analytical and clinical validity. Journal of clinical microbiology. 2012 Apr:50(4):1258-63. doi: 10.1128/JCM.06423-11. Epub 2012 Feb 1
[PubMed PMID: 22301025]
[54]
Otting KA, Stover KR, Cleary JD. Drug-laboratory interaction between beta-lactam antibiotics and the galactomannan antigen test used to detect mould infections. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases. 2014 Sep-Oct:18(5):544-7. doi: 10.1016/j.bjid.2014.03.009. Epub 2014 May 13
[PubMed PMID: 24833197]
[55]
Klingspor L, Loeffler J. Aspergillus PCR formidable challenges and progress. Medical mycology. 2009:47 Suppl 1():S241-7. doi: 10.1080/13693780802616823. Epub 2009 Feb 27
[PubMed PMID: 19253138]
[56]
Guo Y, Bai Y, Yang C, Gu L. Evaluation of Aspergillus IgG, IgM antibody for diagnosing in chronic pulmonary aspergillosis: A prospective study from a single center in China. Medicine. 2019 Apr:98(16):e15021. doi: 10.1097/MD.0000000000015021. Epub
[PubMed PMID: 31008929]
[57]
Mohedano Del Pozo RB, Rubio Alonso M, Cuétara García MS. Diagnosis of invasive fungal disease in hospitalized patients with chronic obstructive pulmonary disease. Revista iberoamericana de micologia. 2018 Jul-Sep:35(3):117-122. doi: 10.1016/j.riam.2017.07.004. Epub 2018 Aug 2
[PubMed PMID: 30078525]
[58]
Kamal YA. Black pleural effusion: etiology, diagnosis, and treatment. Indian journal of thoracic and cardiovascular surgery. 2019 Jul:35(3):485-492. doi: 10.1007/s12055-018-0756-6. Epub 2018 Nov 5
[PubMed PMID: 33061034]
[59]
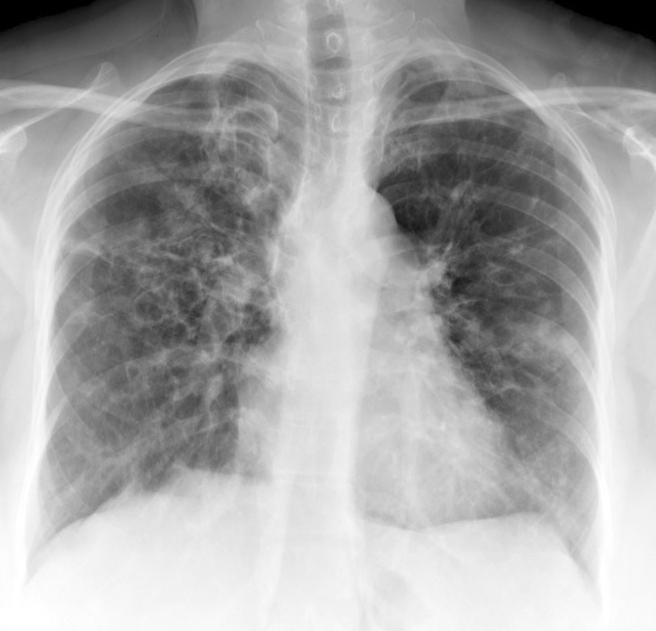
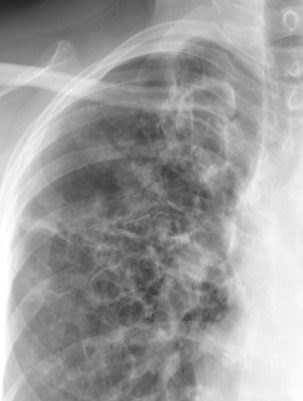
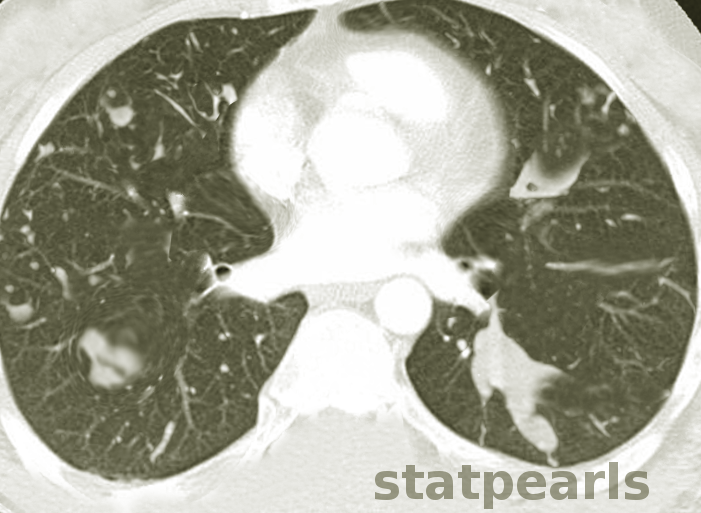
Davda S, Kowa XY, Aziz Z, Ellis S, Cheasty E, Cappocci S, Balan A. The development of pulmonary aspergillosis and its histologic, clinical, and radiologic manifestations. Clinical radiology. 2018 Nov:73(11):913-921. doi: 10.1016/j.crad.2018.06.017. Epub 2018 Jul 31
[PubMed PMID: 30075854]
[60]
Greene R. The radiological spectrum of pulmonary aspergillosis. Medical mycology. 2005 May:43 Suppl 1():S147-54
[PubMed PMID: 16110807]
[61]
Chabi ML, Goracci A, Roche N, Paugam A, Lupo A, Revel MP. Pulmonary aspergillosis. Diagnostic and interventional imaging. 2015 May:96(5):435-42. doi: 10.1016/j.diii.2015.01.005. Epub 2015 Mar 6
[PubMed PMID: 25753544]
[62]
Kosmidis C, Mackenzie A, Harris C, Hashad R, Lynch F, Denning DW. The incidence of cutaneous squamous cell carcinoma in patients receiving voriconazole therapy for chronic pulmonary aspergillosis. Naunyn-Schmiedeberg's archives of pharmacology. 2020 Nov:393(11):2233-2237. doi: 10.1007/s00210-020-01950-x. Epub 2020 Aug 21
[PubMed PMID: 32820348]
[63]
Alastruey-Izquierdo A, Cadranel J, Flick H, Godet C, Hennequin C, Hoenigl M, Kosmidis C, Lange C, Munteanu O, Page I, Salzer HJF, on behalf of CPAnet. Treatment of Chronic Pulmonary Aspergillosis: Current Standards and Future Perspectives. Respiration; international review of thoracic diseases. 2018:96(2):159-170. doi: 10.1159/000489474. Epub 2018 Jul 6
[PubMed PMID: 29982245]
Level 3 (low-level) evidence
[64]
Cornely OA, Koehler P, Arenz D, C Mellinghoff S. EQUAL Aspergillosis Score 2018: An ECMM score derived from current guidelines to measure QUALity of the clinical management of invasive pulmonary aspergillosis. Mycoses. 2018 Nov:61(11):833-836. doi: 10.1111/myc.12820. Epub 2018 Jul 13
[PubMed PMID: 29944740]
Level 2 (mid-level) evidence
[65]
Yun CH, Zhou W, Rui YW. [Classification and surgery of chronic pulmonary aspergillosis]. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases. 2017 Oct 12:40(10):780-782. doi: 10.3760/cma.j.issn.1001-0939.2017.10.017. Epub
[PubMed PMID: 29050136]
[66]
Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B, Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. The New England journal of medicine. 2002 Aug 8:347(6):408-15
[PubMed PMID: 12167683]
[67]
Sam QH, Yew WS, Seneviratne CJ, Chang MW, Chai LYA. Immunomodulation as Therapy for Fungal Infection: Are We Closer? Frontiers in microbiology. 2018:9():1612. doi: 10.3389/fmicb.2018.01612. Epub 2018 Jul 25
[PubMed PMID: 30090091]
[68]
Kwon JC, Kim SH, Park SH, Choi SM, Lee DG, Choi JH, Yoo JH, Kim YJ, Lee S, Kim HJ, Lee JW, Min WS. Prognosis of invasive pulmonary aspergillosis in patients with hematologic diseases in Korea. Tuberculosis and respiratory diseases. 2012 Mar:72(3):284-92. doi: 10.4046/trd.2012.72.3.284. Epub 2012 Mar 31
[PubMed PMID: 23227068]
[69]
Ruhnke M, Kofla G, Otto K, Schwartz S. CNS aspergillosis: recognition, diagnosis and management. CNS drugs. 2007:21(8):659-76
[PubMed PMID: 17630818]
[70]
Baddley JW, Andes DR, Marr KA, Kauffman CA, Kontoyiannis DP, Ito JI, Schuster MG, Brizendine KD, Patterson TF, Lyon GM, Boeckh M, Oster RA, Chiller T, Pappas PG. Antifungal therapy and length of hospitalization in transplant patients with invasive aspergillosis. Medical mycology. 2013 Feb:51(2):128-35. doi: 10.3109/13693786.2012.690108. Epub 2012 Jun 11
[PubMed PMID: 22680976]
[71]
Ben-Ami R, Halaburda K, Klyasova G, Metan G, Torosian T, Akova M. A multidisciplinary team approach to the management of patients with suspected or diagnosed invasive fungal disease. The Journal of antimicrobial chemotherapy. 2013 Nov:68 Suppl 3():iii25-33. doi: 10.1093/jac/dkt390. Epub
[PubMed PMID: 24155143]