Introduction
A ureterocele is a congenital anomaly characterized by the cystic dilatation of the intravesical portion of the distal ureter.[1] The anomaly could affect a single-system kidney, but it primarily affects the upper pole of a duplicated renal unit in 80% of cases.[1][2]
In 1927, Chwalla postulated that ureteroceles are caused by a membrane that occludes the distal ureter (called Chwalla membrane), which is composed of urogenital sinus tissue and ureteric epithelium and fails to dissolve.[3] However, a more complex underlying embryological origin is suggested by the presence of multiple associated anomalies along the course of the Wolffian duct migration.[1][3]
The 2 types of ureteroceles are intravesical and extravesical (ectopic).[4][5][6] Intravesical ureteroceles include stenotic ureteroceles and nonobstructed, large ureteric orifices.[7] They are typically associated with nonduplicated renal systems. Extravesical or ectopic ureteroceles comprise sphincteric ureteroceles, sphincter-stenotic ureteroceles, cecoureterocele, and blind ectopic ureteral anomalies.[4][7] These are more likely to be found in duplicated systems. When ureteroceles are associated with duplicated renal units, about 60% will be extravesical or ectopic.[8]
Intravesical Ureteroceles
Ureteroceles are more commonly discovered in individuals with single renal units. In approximately 40% of stenotic ureterocele cases, the ureteral orifice is relatively small, leading to partial obstruction of the proximal ureter. This condition typically results in some degree of distal ureteral dilation and may impact a duplicated system's kidney or upper pole. On the other hand, about 5% of nonobstructing intravesical ureterocele cases exhibit a large, open ureteral orifice.[2][7]
Extravesical (Ectopic) Ureteroceles
Extravesical (ectopic) ureteroceles are more likely than intravesical ureteroceles to be linked with complete renal duplications. The location of their insertion varies, but it generally follows the migratory path of the Wolffian duct.[1][2][7]
In approximately 40% of cases involving a sphincteric ureterocele, the ureteral orifice may be extravesical, which can either be of normal size or enlarged, extending into the bladder neck and opening anywhere proximal to the external sphincter. In females, the ureterocele meatus may even open beyond the external sphincter. This configuration can obstruct the ureter or the upper pole moiety in completely duplicated systems. When the orifice of the ureterocele is positioned within the internal sphincter, it is more likely to cause proximal dilation due to obstruction.[9] In 5% of cases, a sphincter-stenotic ureterocele is similar to the sphincteric type, but the orifice is obstructed.[1]
Notably, the actual orifice of a sphincteric ureterocele tends to remain open and nonstenotic, primarily influenced by sphincter activity.
In 5% of cases, a sphincter-stenotic ureterocele resembles the sphincteric type but with an obstructed orifice. In another 5% of cases, a blind ectopic ureterocele is similar to the sphincteric ureterocele but lacks a ureteral orifice.
An uncommon type known as a cecoureterocele accounts for 5% of cases. In this variant, the orifice opens within the bladder with a long tubular ureteral extension or a blind pouch that extends into and beyond the bladder neck by tunneling into the submucosa under the trigone. This pouch fills during voiding, causing urethral obstruction and blocking urinary flow. The orifice of the cecoureterocele may be stenotic or open, with stenotic orifices draining exclusively from the kidney, while an open orifice indicates filling from the bladder.[1]
Etiology
During the fourth week of pregnancy, the embryo's kidneys begin forming as the ureteral bud branches off the mesonephric duct and moves toward the metanephric blastema. This usually happens around 32 days after conception. Typically, 1 ureteral bud starts the process of nephrogenesis, resulting in 1 renal pelvis and normal calyces.[10]
Partial ureteral duplication occurs when the ureteral bud splits before reaching the metanephros.[11] The location of this split determines the resulting ureteral anatomy. In cases of partial duplication, both an upper and lower renal unit are present, each with 2 separate ureters proximally. These ureters eventually merge before entering the bladder, with a single ureteral orifice on each side.
Complete duplication will occur if the mesonephric duct produces 2 separate ureteral buds, each connecting with the metanephric blastema.[12] An additional ureteric bud can sometimes develop, resulting in a completely duplicated system per the Meyer-Weigert law. However, some case reports indicate a few rare exceptions to this rule.[13][14][15]
A tendency to form ureteroceles seems to run in families, which suggests a genetic predisposition.[8][16] Ureteroceles are usually isolated abnormalities not associated with other urological congenital anomalies.[17]
Various theories explain ureterocele development. The persistence of the Chwalla membrane (between the ureteral bud and mesonephric duct) can obstruct and lead to ureterocele formation, particularly for typical stenotic intravesical ureteroceles. However, other mechanisms, especially for ectopic ureteroceles, are likely involved. Abnormal development of the trigone's musculature can create an intravesical gap over part of the intramural ureter, potentially leading to a ureterocele. Delayed incorporation of the ureteral bud into the bladder can also interfere with ureteral orifice and intramural ureter development, resulting in a ureterocele.[8][18][19]
Epidemiology
Ureteroceles are a relatively rare congenital anomaly, affecting females 4 to 6 times more frequently than males, partly because completely duplicated systems are also more common in women.[20] Their overall incidence is approximately 1 in 500 to 4000 individuals.[1][21] Notably, this condition appears more prevalent on the left side and in individuals of White racial background.[22]
Ureteroceles involving a single, nonduplicated kidney are more likely to be intravesical than ectopic and are more prevalent in males, accounting for 66% of cases in this gender.[1][20][23] Among women with ureteroceles, 95% will have duplicated systems, while only 34% of men will present with this condition.[1]
About 10% of all ureteroceles are bilateral, potentially affecting both renal units.[20] Among these bilateral cases, approximately 80% are associated with completely duplicated urinary systems; each kidney has 2 separate ureters.[8][20] The remaining 20% of ureteroceles are sporadic anomalies without an associated duplicated urinary system.
Pathophysiology
The Meyer-Weigert law is a fundamental principle in the context of duplicated (duplex) urinary systems. According to this principle, the upper moiety typically drains into the more distal and medial orifice, potentially associated with a ureterocele or an ectopic ureter (a ureter that doesn't enter the bladder at the usual location). This ureter is likely to become obstructed. Conversely, the ureter from the lower moiety typically drains into the more proximal and lateral orifice, which is more likely to develop vesicoureteral reflux.[14] It may be linked to a refluxing ureter in which urine flows backward from the bladder into the ureter.
Consequently, the insufficient intramural ureteral length makes the lower moiety more susceptible to vesicoureteric reflux, as stated by the Paquin rule.[24] Paquin suggested an ideal intramural ureter should be approximately 5 times longer than its diameter to prevent reflux. Consequently, reflux frequently occurs in the ureter, which drains the lower moiety and, therefore, has a much shorter intramural length.
While the Paquin rule has stood the test of time, recent studies have suggested that it overestimates the length required and does not adequately consider the anatomy of the ureteral orifice itself or changes to the intramural ureter caused by bladder filling.[25][26][27] As the bladder fills, there is a deformation of the bladder wall, and the intramural ureter becomes naturally longer and narrower, which would allow a shorter intramural length to suffice.[27][28]
Occasional confusion arises about using ureteral diameter or circumference to calculate the optimal intramural length (it should be diameter). Currently, the minimum acceptable length-to-diameter ratio for surgical ureteral reimplantations is 3:1.
In cases where adequate submucosal tunneling is not possible, an antireflux staple-less split cuff ureteral nipple is an alternative for achieving an antireflux anastomosis.[29][30][31][32] This technique has shown promising results.[29][30][31][32]
Histopathology
Histologically, the walls of a ureterocele are comprised of fibrous tissue and hypoplastic muscle tissue lined with ureteral urothelium and covered by normal bladder mucosa.[16][23]
History and Physical
Undetected or untreated ureteroceles can substantially negatively impact the well-being and quality of life of individuals affected by them. Therefore, performing a thorough medical history assessment during the diagnosis and evaluation of ureteroceles is crucial. This approach is essential for ensuring prompt diagnosis and the potential need for treatment. Additionally, it's important not to overlook family history, as it can increase risk.
Failure to thrive, recurrent or early UTI, and urosepsis are common presenting symptoms of ureteroceles in neonates and children.[8] Urinary symptoms can also develop if an ectopic ureterocele causes urethral obstruction.[33][34][35] If a large ureterocele becomes prolapsed, it may appear as a vaginal mass in females.[16] Other possible presenting symptoms in children include incontinence, urolithiasis, an unexplained abdominal mass, urinary outlet obstruction, and urethral or vaginal prolapse.[21][36]
In adults, the condition may be asymptomatic or cause flank pain, urolithiasis, or recurrent UTIs. A ureterocele may also rarely be a cause of chronic abdominal pain.[37]
The assessment and management of ureteroceles are typically divided into 2 categories based on when the condition becomes evident: antenatal and postnatal. In the antenatal period, routine ultrasound scans conducted at 12 to 14 weeks and again at 20 weeks gestation can often identify ureteroceles.[38] However, the most accurate assessment of the fetal urinary tract typically occurs at 28 weeks gestation. About 2% of all cases of prenatal hydronephrosis are caused by ureteroceles.
Postnatally, a child with ureteroceles may experience symptoms such as pain, UTIs (the most common presentation), or the presence of a bulging cystic swelling associated with the condition.[23] Hydronephrosis with abdominal pain and stones are other common presenting symptoms. Monitoring a baby's weight and height on growth charts and conducting periodic urinalyses are essential because recurrent UTIs or sepsis can have detrimental effects on growth and development.
In adults, ureteroceles are often found incidentally when imaging studies are performed for unrelated reasons. Adult ureteroceles are typically unduplicated, intravesical, and involve a single system.[23]
Vital signs, including pyrexia (fever) and elevated blood pressure, are crucial indicators of condition severity. Pyrexia may suggest a severe infection and the potential development of sepsis. At the same time, hypertension can arise as a long-term complication due to renal scarring resulting from pyelonephritis, especially in children <4 years of age.
On clinical examination, abdominal palpation may reveal tenderness, particularly in the flank area, and occasionally, a mass may be felt. The discovery of a palpable mass in the loin area may be incidental and could indicate a nonfunctioning kidney or renal moiety. A prolapsed ureterocele may appear as a vaginal mass in females.[16][39]
Evaluation
A comprehensive evaluation is essential for the proper identification and management of ureteroceles.[40] This typically involves a range of investigations, including blood and urine tests and radiological assessments. These diagnostic measures are crucial for diagnosing the condition, assessing its severity, estimating potential complications, and determining the most appropriate treatment.[40] Most ureteroceles (90%) are diagnosed before the age of 3 years, and increasing numbers are being diagnosed antenatally.[41][42]
Laboratory Testing
From a laboratory perspective, a series of blood tests is necessary, primarily focusing on conducting a complete blood count, kidney function tests, and measuring C-reactive protein (CRP) levels. Disrupted kidney function may indicate kidney obstruction, while elevated inflammatory markers, such as CRP, can provide valuable insights into the severity of the infection. Furthermore, a urine dipstick test is a straightforward and noninvasive method to identify UTIs. A positive result for nitrates and leukocytes can indicate a UTI, while proteinuria may suggest poor or deteriorating kidney function.
In the event of a positive result for nitrates on the urine dipstick test, it is advisable to send a urine sample for microscopic urinalysis and culture. UTIs often accompany ureteroceles, and this additional testing helps detect specific infective organisms and assess their antibiotic sensitivity, allowing for treatment optimization for the specific bacterium.
Radiological Imaging
Ultrasound is usually sufficient to make an initial, presumptive diagnosis. Ultrasound will demonstrate a cystic, well-defined, rounded mass in the posterior bladder. It is best seen when the bladder is partially filled. (If the bladder is empty, the structure is immediately adjacent to the bladder walls and not distinguishable. If the bladder is too full, the ureterocele may prolapse, or its walls may be compressed, making it hard to visualize.)[43] The distal ureter may contain a stone or appear dilated. Further evaluation and diagnostic tests are generally needed to define the anatomy better and determine the appropriate management of such cases.
Radiologically, investigations for ureteroceles can be categorized into antenatal and postnatal assessments. During the antenatal period, routine ultrasound scans are typically highly sensitive and effective in detecting and identifying significant ureteroceles.[8][44] Following the antenatal detection of ureteroceles, appropriate management is initiated (see Image. Antenatal Ultrasound of Fetal Ureterocele). When prenatal ultrasound identifies a duplicated system with hydronephrosis involving the upper pole, a ureterocele is found 70% of the time.[45]
Repeat postnatal follow-up ultrasound scans should not be performed within the first week to prevent potential false negative results caused by physiological dehydration, which may occur within the initial 48 hours and could lead to misleading reassurance. Some pediatricians prefer to wait 4 to 6 weeks before performing a repeat ultrasound scan as long as the child remains in good health and maintains stable vital signs.
A voiding cystourethrogram (VCUG) should be performed at age 4 to 6 weeks to evaluate the infant for vesicoureteral reflux and delineate the ureterocele.[41][46] In cases of symptomatic children, particularly those who develop severe infections or sepsis due to the condition, performing a voiding cystourethrogram may be conducted at an earlier age. Nevertheless, it is crucial to rule out and treat any UTIs before conducting the study.
Reflux involving the lower pole moiety of a duplicated system with a ureterocele associated with the upper pole segment is found in about 50% of cases on the ipsilateral side and 25% on the contralateral side.[8][42][47][48] Along with ultrasound, VCUG helps identify any ureteral or renal calculi. Too much contrast can make identification and visualization of the ureterocele more difficult, so early images during bladder filling for the VCUG are suggested to optimize ureterocele detection. If reflux is detected, prophylactic antibiotics are generally recommended until this is corrected.
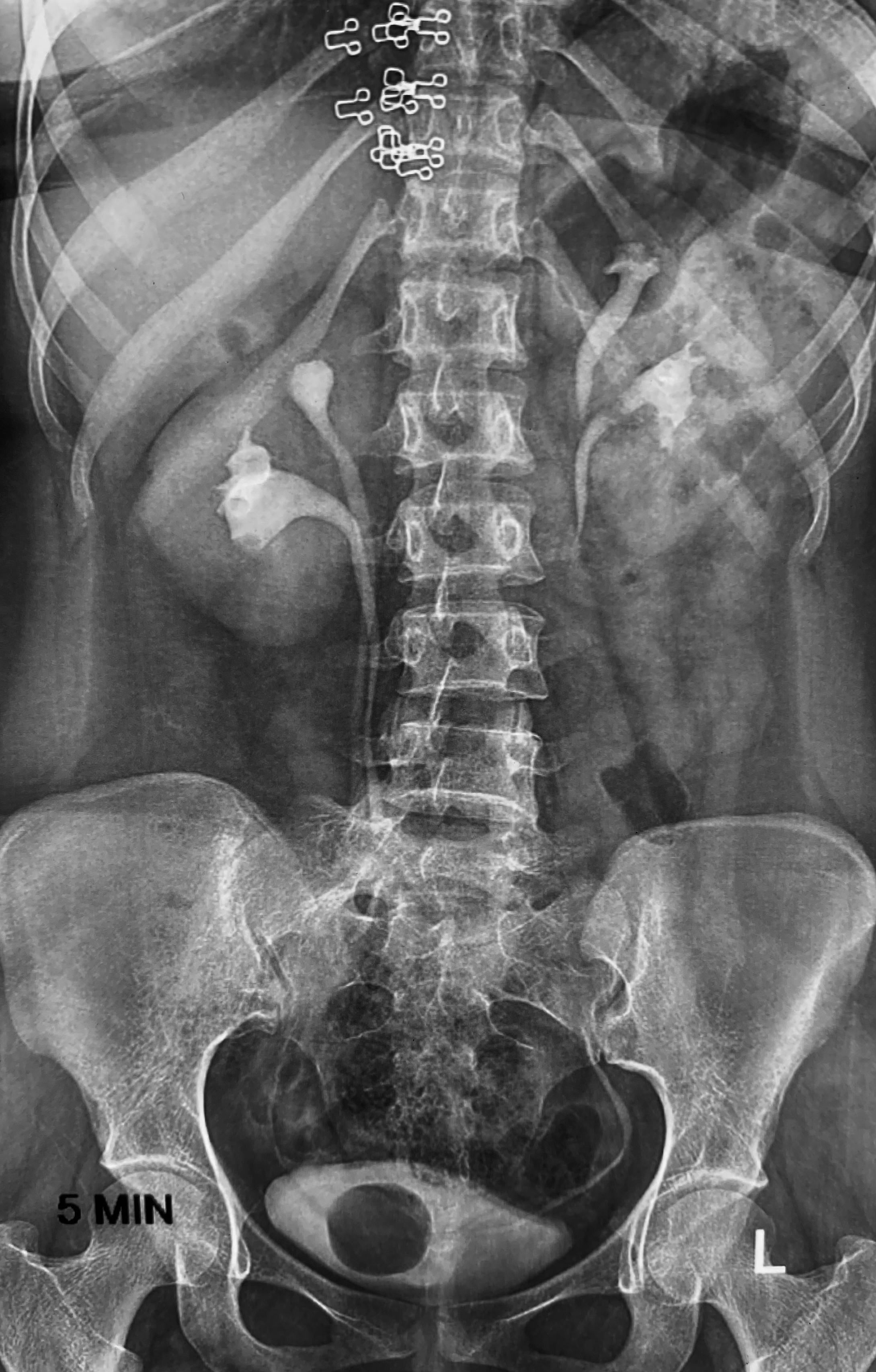
In a VCUG or CT urogram, a ureterocele typically presents as a circular filling defect within the bladder, displaying the distinctive "cobra head sign" (see Image. Intravenous Pyelogram Shows Bilateral Duplex Kidneys).[49] Although no longer the preferred imaging modality, an intravenous pyelogram can also clarify the anatomy of a ureterocele and complex duplicated system and help determine relative renal unit function.[2][41]
If postnatal confirmation reveals hydronephrosis (a condition where the renal pelvis swells due to a buildup of urine), the next steps should include a voiding cystourethrogram to assess the urinary tract for reflux and a mercaptoacetyltriglycine (MAG3) renogram at age 3 months. This timing allows for the maturation of the renal glomeruli and tubules, ensuring the proper development and function of these structures.
The MAG3 renogram is a valuable diagnostic tool that provides insights into the differential kidney function and helps confirm or rule out any obstruction. The renogram can be either a dimercaptosuccinic acid (DMSA) or MAG3 scan, typically performed 3 months after birth. These scans help evaluate and track renal function as well as identify any scarring in the case of DMSA. In cases of recent infection, the renogram should be performed at least 3 months after the infection has subsided. This precaution helps ensure that the results are not affected by the residual effects of the infection, minimizing the risk of misleading findings.[50][51]
The renogram uses a radioactive tracer, technetium m99, with a relatively short half-life of 6 hours.[52] The renogram can provide differential renal functional percentages, which are useful for tracking kidney growth and function, as well as helping determine the relative value of performing reconstructive surgery versus a nephrectomy (partial or total) for poorly functioning renal units or upper pole moieties. In general, less than 10% function in a renal unit is not considered worth saving, and a partial nephrectomy is likely to be recommended.[53]
Magnetic resonance urography (MRU) is another valuable adjunct in evaluating ureteroceles and related urinary tract anomalies. MRU is particularly beneficial in cases involving complex duplication patterns and when assessing the function of a dysplastic upper pole, especially when associated with an infrasphincteric ectopic ureter. In addition, the advantage of avoiding radiation exposure has led to MRU rapidly becoming the preferred diagnostic tool for girls experiencing urinary incontinence suspected to be linked to an ectopic ureter or ureterocele.
Moreover, MRUs offer precise anatomical and vascular delineation while providing insights into renal function. However, MRU can be relatively expensive, and in many cases, it necessitates the use of sedation or general anesthesia to ensure that the infant remains still and cooperative during the scan.[52]
For conservatively managed cases of ureteroceles, while specific guidelines may not provide exact dates for follow-up scans, it is typically recommended to conduct ultrasound scans every 6 months and MAG3 scans yearly or more frequently if ultrasound results indicate deterioration. These regular imaging assessments help monitor the condition, assess any changes, and ensure appropriate management decisions are made over time. Following an endoscopic ureterocele incision, it is common practice to repeat both an ultrasound and a cystourethrogram scan postoperatively to assess the procedure's results.
Cystoscopy
Notably, while not routinely required, cystoscopy can be a valuable tool for diagnosing and managing specific cases. For instance, in selected patients, visual assessment through cystoscopy (and vaginoscopy) can offer essential additional information about ureteroceles that may not have been evident through diagnostic imaging alone. Furthermore, cystoscopy may occasionally reveal an occult ectopic ureteral orifice, contributing to a more comprehensive understanding of the anatomy and guiding appropriate treatment decisions.
Treatment / Management
The management of ureteroceles can be complex and multifaceted, with various factors influencing the chosen approach. These factors include the patient's age, the severity of the condition, the relative renal function of each kidney or segment, clinical symptoms, the health of the contralateral kidney, the presence or absence of vesicoureteral reflux, and the specific type of ureterocele (intravesical or ectopic).[54]
The primary objective is typically to assess whether treatment is necessary, what form it should take, and when it should be administered. Ultimately, the overarching goal is to preserve kidney function, avoid recurrent infections, maintain continence, control symptoms, eliminate vesicoureteral reflux, and optimize quality of life by tailoring the management plan to the individual needs and circumstances of the patient.[1][55][56]
Preserving the function of the upper moiety in cases involving a duplex urinary system by early endoscopic incision is paramount. At the same time, antibiotic prophylaxis is generally used for vesicoureteral reflux until it resolves or definitive treatment is implemented. In duplicated systems, the upper pole is responsible for only up to one-third of that kidney's total function, and permanent damage is found on pathological examination in up to 92% of resected upper pole segments.[57] Therefore, in many cases, extensive surgery and extreme measures to preserve such marginal function are not logical, beneficial, or advantageous.[1]
In cases where ureteroceles are asymptomatic, there is no vesicoureteral reflux, and kidney function is preserved, it is often advisable not to intervene, especially since refluxing sterile urine is unlikely to harm the kidney.[8][55][58] In such situations, a MAG3 renogram is needed to assess the differential function of the kidneys and rule out any potential obstruction.[58][59]
Antenatal and postnatal care for ureteroceles can vary depending on the specific circumstances and condition severity.
Postnatally discovered ureteroceles may be managed through active surveillance in cases where the infant's symptoms are well-controlled, kidney function is preserved, and there is no bladder-neck obstruction, particularly in larger ureteroceles. Decisions should be tailored to individual clinical factors, ensuring optimal kidney health and symptom management.
Prophylactic antibiotics should be carefully considered in pediatric cases, recognizing the potential for increased antimicrobial resistance. However, this approach can effectively lower the incidence of UTIs, particularly in recurrent pyelonephritis associated with a refluxing ureterocele. This approach should be weighed against the risks and benefits for each patient.
The first 4 years of life are typically the most crucial in managing urinary infections associated with ureteroceles, as untreated infections during this period can potentially lead to scarring of renal tissue, ultimately resulting in a loss of function in that part of the upper moiety of the kidney.[60][61]
Surgical Options
Curative management options include endoscopic incision of the ureterocele, ureteric reimplantation, partial nephroureterectomy, or complete reconstruction.[8][50]
In many cases, initial treatment with an endoscopic incision is performed to prevent UTIs and protect the renal parenchyma by eliminating hydronephrosis and ureteral obstruction.[62] Meanwhile, the infant is managed with prophylactic antibiotics until 1 year of age, when definitive surgery, if needed, is technically far easier.[63] Patients who present as older children are more likely to be initially treated with bladder reconstruction and ureteral reimplantation.
Endoscopic Techniques
Endoscopic techniques are commonly employed for the surgical management of ureteroceles and can also be used to correct vesicoureteral reflux.[64][65][66][67] These minimally invasive approaches offer several advantages, including reduced surgical trauma, shorter recovery times, and potentially improved outcomes, making them valuable options for treating these abnormal urinary tract conditions. In most cases, endoscopic incision is generally considered an excellent treatment choice, especially for initial management.
This procedure, performed using tools such as a Collins knife, resectoscope loop, Bugbee electrode, or laser, involves incising the lower anterior wall of the intravesical ureterocele transversely to create a flap, which helps minimize creating iatrogenic reflux.[54] The incision should be longitudinal for ectopic ureteroceles to avoid leaving a distal lip or pouch and extend through the bladder neck.[54] Alternatively, a laser can be used to create multiple punctures through the wall of the ureterocele.[54] This can be done over a ureteral catheter or stent.[54]
Most pediatric urologists recommend endoscopic incision in cases of symptomatic ureteroceles or when bladder neck obstruction interferes with normal voiding function.[23][54][68][69][70] When used in appropriate cases, studies have indicated a definitive cure in over 90%, although a few reports have suggested that early endoscopic incision of ectopic ureteroceles in children may not always yield the best results in every case.[4][54][68][69][70][71]
The use of a laser puncture technique seems to produce good results with a lower incidence of reflux compared to electrosurgical endoscopic incision, according to a recent meta-analysis and other studies comparing the 2 techniques.[64][72][73]
Endoscopic incision is significantly less successful than the definitive curative therapy for ectopic ureteroceles, with a reported overall success rate of 25% to 30% in these cases.[74][75][76] Larger ureteroceles and those with minimal muscular tissue backing are more likely to demonstrate postoperative reflux. They may require a second procedure to repair if it does not resolve over time as the child grows.[1] Antibiotic prophylaxis should be used while the reflux remains.
Prenatal diagnosis and early endoscopic incisional therapy were associated with better overall outcomes and results than ureterocele patients who were only diagnosed postnatally.[38][77]
Endoscopic incision of ureteroceles is a relatively simple surgical option but is associated with a risk of reflux, which is why thorough follow-up is strongly recommended, including imaging to screen for vesicoureteral reflux.[78] This follow-up typically includes sending urine for microscopic urinalyses and culture, conducting an ultrasound scan of the abdomen and pelvis, and performing a radionuclide renogram and/or a VCUG as appropriate.[78]
Obstructive ureteroceles in adults have been successfully treated endoscopically with dilation utilizing 2 separate double J stents left in place for 6 weeks.[79] This technique minimizes the risk of reflux and avoids the need for more invasive surgery.[79]
Each case should be evaluated individually, but an endoscopic incision is a viable and recommended initial therapy for most symptomatic cases or where renal growth/function may be at risk.[50] Early endoscopic incision of ureteroceles preserves renal function in the associated renal segment, eliminates hydronephrosis, reduces the risk of UTIs and pyelonephritis, and restores the affected ureter to normal size, facilitating ureteral reimplantation if that becomes necessary. Reevaluation of the patient at 1 year with repeat imaging (ultrasound, VCUG, CT scan, and/or renal nuclear scanning as appropriate) can then be used to help determine the need and type of additional surgical therapy necessary.
Other Surgical Options
In cases where a ureterocele incision is not feasible or if the ureterocele is sufficiently large or refluxing, parents can be counseled about the alternative option of ureteral reimplantation. This procedure may also be recommended as a viable surgical approach to address a ureterocele associated with a functional upper pole renal moiety and a severely refluxing lower pole ureter.[53][80] After dissection from the bladder, the 2 ureters are reimplanted together. (Limiting unnecessary ureteral dissection helps preserve their blood supply.)
In 1 study, lower urinary tract reconstruction, when used for ectopic ureterocele repairs in patients with complete duplications, has demonstrated excellent long-term results in experienced hands, with more than 90% of patients demonstrating clinical success. However, about 27% had lower urinary tract dysfunction.[81]
A meta-analysis has demonstrated that following primary ureterocele incision, individuals with an ectopic ureterocele tend to have higher reoperation rates than those with intravesical lesions.[50][51]
Ureterovesical implantation can be performed using either an intravesical (the Leadbetter-Politano or Cohen techniques) or an extravesical approach, such as the Lich-Gregoir procedure. The technical details of these ureteral reimplantation procedures are better described in our companion StatPearls reference review article on Vesicoureteral Reflux.[46]
In cases where an ectopic ureterocele with severe hydronephrosis makes traditional ureteric reimplantation challenging, alternative reconstruction options should be considered. These options may involve procedures like ureteroureterostomy and pyeloureterostomy. Ureteroureterostomies work best when there is no significant disparity between the sizes of the 2 ureters. For this reason, it is usually recommended that the ureteroureterostomy be performed distally, near the bladder.[1]
In instances where renal function loss is significant, as indicated by a renogram showing a split function of 10% or less, upper moiety nephroureterectomy becomes a viable option. This procedure can be performed through open surgery, laparoscopically, or robotically, particularly in cases where symptomatic issues such as pain or recurrent UTIs persist. However, conservative management remains a valid and appropriate approach if the patient remains asymptomatic.
Patients who present with urosepsis associated with obstructive ureteroceles require immediate surgical drainage, usually by urgent endoscopic incision.[74][82] Percutaneous drainage can be performed but is generally not required.
The reconstruction procedure for ureteroceles can be complex and challenging, but when performed by skilled and experienced surgeons, it can potentially preserve optimal kidney function and manage any associated reflux.[59][83][1]
Ureteroceles, Prenatal Hydronephrosis, and Oligohydramnios
In cases of severe prenatal hydronephrosis, especially when bilateral ureteroceles and oligohydramnios (low levels of amniotic fluid) are present during pregnancy, limited research on antenatal treatment has been conducted. However, severe oligohydramnios can potentially impact fetal lung development, and a baby born with severe bilateral hydronephrosis may be at risk for respiratory distress and renal compromise. In such cases, the medical team may need to closely monitor the pregnancy and consider interventions to optimize the baby's lung development and overall health. This could be fatal if the infant is not born in a hospital equipped to manage such cases.
In cases of severe oligohydramnios associated with ureteroceles, prompt medical attention and delivery in a well-equipped hospital are essential to prevent potentially life-threatening complications. Recent interventions have shown promise in addressing this condition antenatally through ultrasound-guided needle ablation, often utilizing laser techniques. However, such procedures are still considered investigational and should only be performed in tertiary care centers as part of a research protocol after fully discussing the significant risks.[84][85][86]
Summary: Guide to Definitive Surgical Treatment Selection for Ureteroceles
Critical issues include presenting symptoms, patient age at the time of diagnosis, contralateral kidney function, intravesical versus ectopic presentation, presence of renal duplication, upper pole moiety function, and vesicoureteral reflux.[8][50]
In cases where the upper pole segment demonstrates good function, and the lower pole is normal without reflux, it is advisable to consider either an ipsilateral ureteroureterostomy or ureteropyelostomy procedure. On the other hand, if hydronephrosis or renal cortical thinning is detected in conjunction with an obstructing ureterocele, early intervention with an endoscopic incision or laser treatment is recommended.
When significant reflux is identified in a lower pole ureteral segment or following an endoscopic incision of a ureterocele, initial management often involves prophylactic antibiotics. Definitive surgical treatment typically entails bladder reconstruction, including the resection of the ureterocele and reimplantation of both ipsilateral ureters as a combined procedure.
A heminephrectomy is indicated when the upper pole moiety exhibits poor relative function, typically less than 10%. This procedure can be performed using open, laparoscopic, or robotic techniques. Antibiotic prophylaxis may be considered until definitive surgery, which is often postponed until the infant reaches 1 year of age.
Differential Diagnosis
The differential diagnoses for ureteroceles include the following:
- Bladder diverticulum ("Hutch" or periurethral)
- Cystitis
- Functional abdominal pain
- Nephrolithiasis
- Pelvic organ prolapse
- Pseudoureterocele
- Pyelonephritis
- UTI
- Vesicoureteral reflux
Prognosis
The prognosis of ureteroceles is influenced by various factors, including the condition's severity, complexity, and the timing of diagnosis. In extreme cases or when the diagnosis is delayed, the risk of significant morbidity, including potential kidney function loss or even fatal outcomes in cases of uncontrolled sepsis, can result.
Conversely, excellent outcomes can be achieved if ureteroceles are promptly diagnosed and managed. Timely recognition and intervention are critical in optimizing the prognosis for individuals with ureteroceles.
Complications
Ureteroceles can lead to various complications, depending on the severity and management of the condition. Some potential complications include those listed below.
- Hydronephrosis: In severe cases, untreated ureteroceles can result in the enlargement of the affected kidney, a condition known as hydronephrosis, due to urine buildup.
- Hypertension: Persistent kidney issues can lead to high blood pressure (hypertension), which may require management.
- Lower urinary tract voiding dysfunction (especially in extravesical types): Voiding symptoms, including incontinence, frequency, or urgency, may be transient, intermittent, or persistent.
- Obstruction: Ureteroceles may cause partial or complete urinary tract obstruction, impairing urine flow from the kidney to the bladder.
- Pain: Ureteroceles can cause abdominal or flank pain, especially in symptomatic cases.
- Psychosocial impact and developmental delays: In children, frequent infections, medical procedures, or surgical interventions related to ureteroceles can have psychosocial implications and developmental delays.
- Recurrent UTIs: Ureteroceles can predispose individuals, especially children, to recurrent UTIs due to urine reflux or urinary stasis within the ureterocele.
- Renal function impairment: Prolonged obstruction or recurrent infections can lead to kidney damage and a decline in renal function, primarily if not managed appropriately.
- Renal scarring: Chronic or severe infections can cause scarring in the kidney tissue, potentially affecting kidney function.
- Sepsis: In severe cases, untreated or recurrent UTIs associated with ureteroceles can lead to life-threatening sepsis.
- Stones: Calculi can form in the dilated ureterocele, or a renal/ureteral stone can become trapped in the ureterocele cavity.
- Vesicoureteral reflux: This may develop as a postoperative complication from endoscopic incision of the ureterocele and lead to recurrent infections and diminished renal growth or function.
Diagnosing and managing ureteroceles promptly to prevent or minimize these potential complications and preserve kidney function and overall health are essential. The specific complications can vary depending on individual factors and the type of ureterocele.
Deterrence and Patient Education
Antenatal screening plays a vital role in the early detection of ureteroceles, and parents should be fully educated about the child's clinical condition. More importantly, they should be taught about early signs of complications such as infection (UTIs or pyelonephritis), pain, and when to seek urgent medical assistance.
Antenatal screening is crucial in the early detection of ureteroceles, and it serves as an opportunity to educate parents about their child's clinical situation and requirements after birth, including the potential need for surgical intervention. Most importantly, parents should be educated about recognizing the early signs of complications, such as pain and infections resulting in UTIs or pyelonephritis, and the need to seek urgent medical consultation.
Timely awareness and action can be instrumental in ensuring the child's health and preventing complications associated with ureteroceles.
Patient Education
When discussing ureteroceles with parents, it's essential to convey the possibility of developmental delays and the potential need for prophylactic antibiotics. Parents should be informed about the importance of monitoring their child's development and maintaining growth chart records. In the context of managing ureteroceles, urotherapy education is essential.
Parents should be educated about normal bladder function and the significance of regular voiding. This knowledge is crucial, especially in cases of conservative treatment or while awaiting curative management. Urotherapy teachings help parents understand how to support their child's bladder health and contribute to overall treatment effectiveness.
In some instances, prophylactic antibiotic use may be necessary, and it's important to counsel parents about the balance between the risk of antimicrobial resistance and the benefits of preventing infection and sepsis. Parents should also be equipped with practical skills, including collecting urine samples when needed and monitoring bowel activity. These skills and informed decision-making are essential when caring for a child with ureteroceles, ensuring their health and well-being while minimizing potential risks.
Pearls and Other Issues
Ureteroceles are frequently associated with stones due to urinary stasis and/or infection, with an incidence reported as high as 39%.[21][36][87][88] These are usually easily managed with endoscopic incision of the ureterocele, especially in adults.[89][90]
Enhancing Healthcare Team Outcomes
Management decisions for ureteroceles are never clear-cut, and there are situations where the best approach may be subject to debate. Early detection through antenatal ultrasound scans plays a crucial role in facilitating management.[77] Timely recognition allows healthcare providers to assess the condition, determine the appropriate treatment or surveillance strategy, and optimize outcomes for individuals with ureteroceles. Early intervention can significantly impact the long-term health and well-being of affected patients.
Collaboration among various healthcare teams is of paramount importance in the management of ureteroceles. This collaborative effort should begin with the obstetric team during pregnancy, continue through the care provided by pediatric specialists, and involve urology teams as needed. This multidisciplinary approach ensures that the condition is addressed comprehensively, from antenatal screening to postnatal care, enabling the best possible outcomes for individuals with ureteroceles. A multidisciplinary approach allows a comprehensive approach, involving the assessment, diagnosis, and treatment, all working together to enhance the patient's overall health and well-being.
The limited availability of large-scale clinical trials for ureteroceles results in a relatively weak consensus in their management and level of evidence. It's important to acknowledge that ureteroceles are commonly associated with complete renal duplication but can also occur in a single urinary system. Typically, diagnosis occurs in the early years of life through ultrasound imaging, whereas in older children, clinical symptoms often drive the need for assessment and evaluation.[91] The clinical understanding and management of ureteroceles continue to evolve based on clinical experience and smaller-scale studies due to the condition's rarity.
In diagnosing and evaluating ureteroceles, several diagnostic modalities may be employed, including the following:
- Cystoscopy: An endoscopic procedure that allows direct visualization of the ureterocele and the urinary tract.
- High-resolution MRI: Offers enhanced visualization of ureteroceles and associated structures.
- MRU: A noninvasive imaging technique that provides detailed anatomical and functional urinary tract information.
- Radionuclide studies: These include both MAG3 and DMSA renal scans, which assess relative renal function and scarring.
- Ultrasound: Used for early detection, especially in infants and young children (Image. Ultrasound of Bilateral Ureteroceles).
- VCUG: An x-ray imaging procedure to assess bladder and urethral function during urination. It is especially useful for identifying vesicoureteral reflux.
These diagnostic modalities are selected based on the patient's age, clinical presentation, and the need for detailed assessment to guide treatment decisions for ureteroceles.
Management options for ureteroceles range from conservative strategies to minimally invasive procedures like endoscopic decompression or more invasive surgeries such as partial nephroureterectomy or complete primary reconstruction. The choice of approach hinges on multiple factors, including the patient's clinical condition, age, upper pole kidney function, the presence of reflux or obstruction, the existence of bladder-neck obstruction, and whether the ureterocele is intravesical or ectopic. Customized treatment decisions are designed to maximize patient outcomes by preserving kidney function and minimizing complications, taking into account individual clinical factors.[60][92][93]