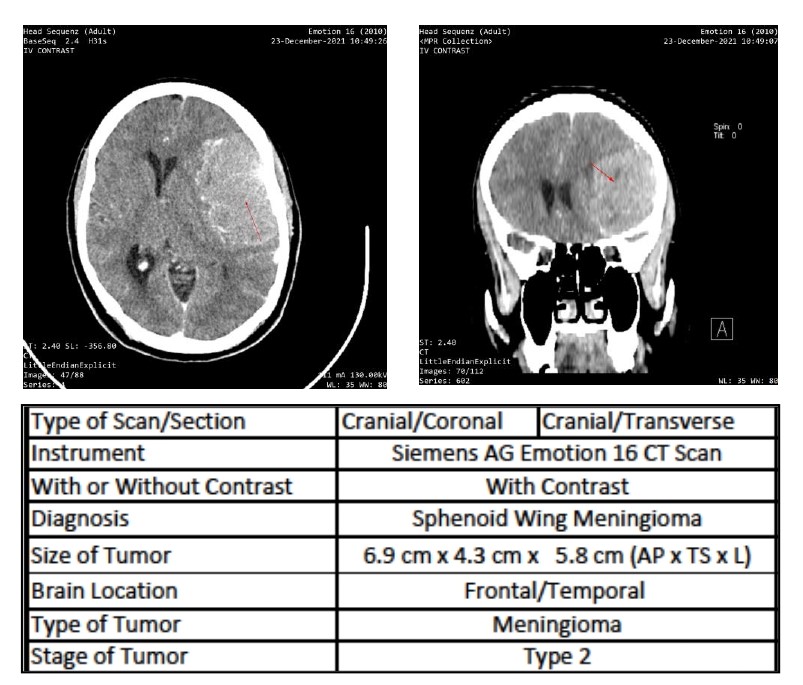
[1]
Hata M, Miyamoto K. Causes and Prognosis of Unilateral and Bilateral Optic Disc Swelling. Neuro-ophthalmology (Aeolus Press). 2017 Aug:41(4):187-191. doi: 10.1080/01658107.2017.1299766. Epub 2017 Apr 10
[PubMed PMID: 29344057]
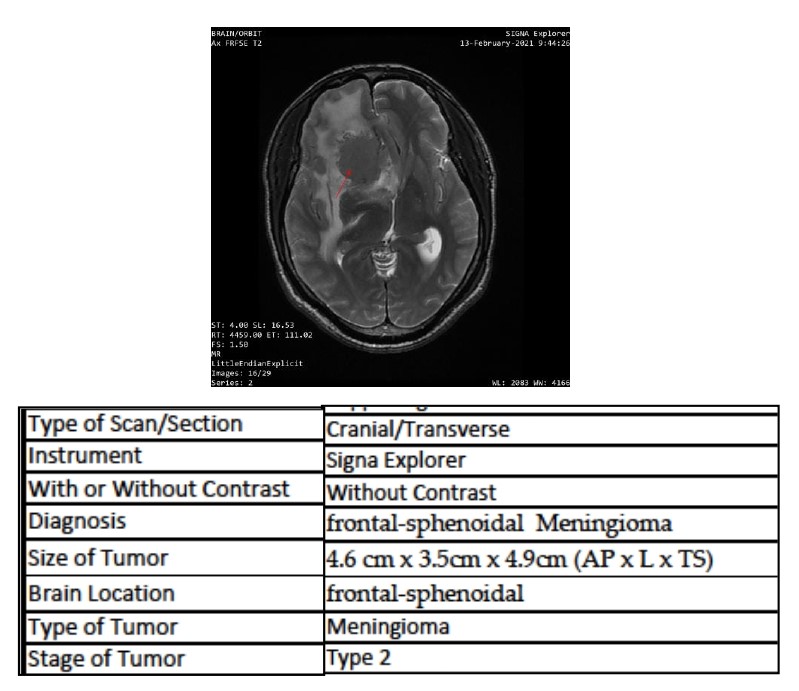
[2]
Eidet JR, Biernat D, Dahlberg D, Wiedmann MKH, Jørstad ØK. [Foster Kennedy Syndrome]. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 2019 Jun 25:139(10):. doi: 10.4045/tidsskr.18.0719. Epub 2019 Jun 24
[PubMed PMID: 31238653]
[3]
Zehden J, Harish Bindiganavile S, Bhat N, Lee AG. Compressive Optic Disc Edema and Contralateral Papilledema: Type 2 Foster Kennedy Variant Syndrome. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2021 Jun 1:41(2):e217-e219. doi: 10.1097/WNO.0000000000001091. Epub
[PubMed PMID: 33105415]
[4]
David C, Suvac E, Tăbăcaru B, Stanca TH. Pseudo-Foster Kennedy Syndrome - a case report. Romanian journal of ophthalmology. 2016 Oct-Dec:60(4):270-274
[PubMed PMID: 29450361]
Level 3 (low-level) evidence
[5]
Micieli JA, Al-Obthani M, Sundaram AN. Pseudo-Foster Kennedy syndrome due to idiopathic intracranial hypertension. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2014 Aug:49(4):e99-e102. doi: 10.1016/j.jcjo.2014.05.002. Epub 2014 Jul 16
[PubMed PMID: 25103671]
[6]
Visa Reñé N, Paredes Carmona F. Pseudo-Foster Kennedy syndrome due to idiopathic intracranial hypertension. Archivos de la Sociedad Espanola de Oftalmologia. 2019 Dec:94(12):598-601. doi: 10.1016/j.oftal.2019.09.006. Epub 2019 Oct 18
[PubMed PMID: 31635921]
[7]
Desai N, Yong RL, Doshi A, Rucker JC. Pseudo-Foster-Kennedy syndrome with optic nerve compression by the gyrus rectus. Neurology. 2015 Jul 28:85(4):385. doi: 10.1212/WNL.0000000000001791. Epub
[PubMed PMID: 26215878]
[8]
Bansal S, Dabbs T, Long V. Pseudo-Foster Kennedy Syndrome due to unilateral optic nerve hypoplasia: a case report. Journal of medical case reports. 2008 Mar 18:2():86. doi: 10.1186/1752-1947-2-86. Epub 2008 Mar 18
[PubMed PMID: 18348732]
Level 3 (low-level) evidence
[9]
Ogasawara C, Philbrick BD, Adamson DC. Meningioma: A Review of Epidemiology, Pathology, Diagnosis, Treatment, and Future Directions. Biomedicines. 2021 Mar 21:9(3):. doi: 10.3390/biomedicines9030319. Epub 2021 Mar 21
[PubMed PMID: 33801089]
Level 3 (low-level) evidence
[10]
Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future oncology (London, England). 2018 Sep:14(21):2161-2177. doi: 10.2217/fon-2018-0006. Epub 2018 Aug 7
[PubMed PMID: 30084265]
Level 3 (low-level) evidence
[11]
Zhang J, Sai K, Zhu ZQ, Lin FH, Wang ZF, Chen YM, Huang CY, Ye YL, Wang XL, Li YP, Sun SX, Zhong WY, Chen JB, Yang YQ. Prognostic factors for olfactory groove meningioma with nasal cavity extension. Oncotarget. 2018 Jan 12:9(4):4607-4613. doi: 10.18632/oncotarget.23461. Epub 2017 Dec 19
[PubMed PMID: 29435128]
[12]
Sun C, Dou Z, Wu J, Jiang B, Iranmanesh Y, Yu X, Li J, Zhou H, Zhong C, Peng Y, Zhuang J, Yu Q, Wu X, Yan F, Xie Q, Chen G. The Preferred Locations of Meningioma According to Different Biological Characteristics Based on Voxel-Wise Analysis. Frontiers in oncology. 2020:10():1412. doi: 10.3389/fonc.2020.01412. Epub 2020 Aug 21
[PubMed PMID: 32974148]
[13]
Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro-oncology. 2019 Nov 1:21(Suppl 5):v1-v100. doi: 10.1093/neuonc/noz150. Epub
[PubMed PMID: 31675094]
[14]
Gatto F, Perez-Rivas LG, Olarescu NC, Khandeva P, Chachlaki K, Trivellin G, Gahete MD, Cuny T, on behalf of the ENEA Young Researchers Committee (EYRC). Diagnosis and Treatment of Parasellar Lesions. Neuroendocrinology. 2020:110(9-10):728-739. doi: 10.1159/000506905. Epub 2020 Mar 4
[PubMed PMID: 32126547]
[15]
Ayele B, Mengesha A, Wotiye A, Alemayehu Y. Giant Pituitary Adenoma Presenting with Foster-Kennedy Syndrome in a 21-Year Old Ethiopian Patient: A Rarely Reported Phenomenon: A Case Report. Ethiopian journal of health sciences. 2020 Mar:30(2):311-314. doi: 10.4314/ejhs.v30i2.19. Epub
[PubMed PMID: 32165821]
Level 3 (low-level) evidence
[16]
Santoro A, Minniti G, Paolini S, Passacantilli E, Missori P, Frati A, Cantore GP. Atypical tentorial meningioma 30 years after radiotherapy for a pituitary adenoma. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2002 Mar:22(6):463-7
[PubMed PMID: 11976979]
[17]
Shashidhar A, Arimappamagan A, Madhusudhan N, Narasinga Rao KVL, Bhat D, Shukla D, Arvinda HR, Srinivas D, Indira Devi B, Somanna S. Transcranial approach for pituitary adenomas - An evaluation of surgical approaches over two decades and factors influencing peri-operative morbidity. Clinical neurology and neurosurgery. 2021 Jan:200():106400. doi: 10.1016/j.clineuro.2020.106400. Epub 2020 Dec 5
[PubMed PMID: 33341089]
[18]
Wadud SA, Ahmed S, Choudhury N, Chowdhury D. Evaluation of ophthalmic manifestations in patients with intracranial tumours. Mymensingh medical journal : MMJ. 2014 Apr:23(2):268-71
[PubMed PMID: 24858153]
[19]
Osaguona VB, Kayoma DH. Etiology of Optic Disc Swelling in a Tertiary Care Center in Nigeria. Nigerian journal of clinical practice. 2020 Dec:23(12):1690-1694. doi: 10.4103/njcp.njcp_333_20. Epub
[PubMed PMID: 33355822]
[20]
Sun T, Plutynski A, Ward S, Rubin JB. An integrative view on sex differences in brain tumors. Cellular and molecular life sciences : CMLS. 2015 Sep:72(17):3323-42. doi: 10.1007/s00018-015-1930-2. Epub 2015 May 19
[PubMed PMID: 25985759]
[21]
Pastora-Salvador N, Peralta-Calvo J. Foster Kennedy syndrome: papilledema in one eye with optic atrophy in the other eye. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2011 Dec 13:183(18):2135. doi: 10.1503/cmaj.101937. Epub 2011 Sep 26
[PubMed PMID: 21948724]
[22]
Kotecha M, Gotecha S, Chugh A, Punia P. Neuroophthalmic Manifestations of Intracranial Tumours in Children. Case reports in ophthalmological medicine. 2021:2021():7793382. doi: 10.1155/2021/7793382. Epub 2021 May 15
[PubMed PMID: 34055437]
Level 3 (low-level) evidence
[23]
Brusini P, Salvetat ML, Zeppieri M. How to Measure Intraocular Pressure: An Updated Review of Various Tonometers. Journal of clinical medicine. 2021 Aug 27:10(17):. doi: 10.3390/jcm10173860. Epub 2021 Aug 27
[PubMed PMID: 34501306]
[24]
Li Z, Yang Y, Lu Y, Liu D, Xu E, Jia J, Yang D, Zhang X, Yang H, Ma D, Wang N. Intraocular pressure vs intracranial pressure in disease conditions: a prospective cohort study (Beijing iCOP study). BMC neurology. 2012 Aug 3:12():66
[PubMed PMID: 22862817]
[25]
Solbak MS, Henning MK, England A, Martinsen AC, Aaløkken TM, Johansen S. Impact of iodine concentration and scan parameters on image quality, contrast enhancement and radiation dose in thoracic CT. European radiology experimental. 2020 Sep 11:4(1):57. doi: 10.1186/s41747-020-00184-z. Epub 2020 Sep 11
[PubMed PMID: 32915405]
Level 2 (mid-level) evidence
[26]
Iyer VR, Ehman EC, Khandelwal A, Wells ML, Lee YS, Weber NM, Johnson MP, Yu L, McCollough CH, Fletcher JG. Image quality in abdominal CT using an iodine contrast reduction algorithm employing patient size and weight and low kV CT technique. Acta radiologica (Stockholm, Sweden : 1987). 2020 Sep:61(9):1186-1195. doi: 10.1177/0284185119898655. Epub 2020 Jan 27
[PubMed PMID: 31986894]
Level 2 (mid-level) evidence
[27]
Sohn SY, Choi JH, Kim NK, Joung JY, Cho YY, Park SM, Kim TH, Jin SM, Bae JC, Lee SY, Chung JH, Kim SW. The impact of iodinated contrast agent administered during preoperative computed tomography scan on body iodine pool in patients with differentiated thyroid cancer preparing for radioactive iodine treatment. Thyroid : official journal of the American Thyroid Association. 2014 May:24(5):872-7. doi: 10.1089/thy.2013.0238. Epub 2014 Mar 6
[PubMed PMID: 24295076]
[28]
Ho JD, Tsang JF, Scoggan KA, Leslie WD. Urinary Iodine Clearance following Iodinated Contrast Administration: A Comparison of Euthyroid and Postthyroidectomy Subjects. Journal of thyroid research. 2014:2014():580569. doi: 10.1155/2014/580569. Epub 2014 Nov 12
[PubMed PMID: 25478285]
[29]
Power SP, Moloney F, Twomey M, James K, O'Connor OJ, Maher MM. Computed tomography and patient risk: Facts, perceptions and uncertainties. World journal of radiology. 2016 Dec 28:8(12):902-915. doi: 10.4329/wjr.v8.i12.902. Epub
[PubMed PMID: 28070242]
[30]
Walter FM, Penfold C, Joannides A, Saji S, Johnson M, Watts C, Brodbelt A, Jenkinson MD, Price SJ, Hamilton W, Scott SE. Missed opportunities for diagnosing brain tumours in primary care: a qualitative study of patient experiences. The British journal of general practice : the journal of the Royal College of General Practitioners. 2019 Apr:69(681):e224-e235. doi: 10.3399/bjgp19X701861. Epub 2019 Mar 11
[PubMed PMID: 30858332]
Level 2 (mid-level) evidence
[31]
Zienius K, Chak-Lam I, Park J, Ozawa M, Hamilton W, Weller D, Summers D, Porteous L, Mohiuddin S, Keeney E, Hollingworth W, Ben-Shlomo Y, Grant R, Brennan PM. Direct access CT for suspicion of brain tumour: an analysis of referral pathways in a population-based patient group. BMC family practice. 2019 Aug 20:20(1):118. doi: 10.1186/s12875-019-1003-y. Epub 2019 Aug 20
[PubMed PMID: 31431191]
[32]
Grover VP, Tognarelli JM, Crossey MM, Cox IJ, Taylor-Robinson SD, McPhail MJ. Magnetic Resonance Imaging: Principles and Techniques: Lessons for Clinicians. Journal of clinical and experimental hepatology. 2015 Sep:5(3):246-55. doi: 10.1016/j.jceh.2015.08.001. Epub 2015 Aug 20
[PubMed PMID: 26628842]
[33]
Frankel J, Wilén J, Hansson Mild K. Assessing Exposures to Magnetic Resonance Imaging's Complex Mixture of Magnetic Fields for In Vivo, In Vitro, and Epidemiologic Studies of Health Effects for Staff and Patients. Frontiers in public health. 2018:6():66. doi: 10.3389/fpubh.2018.00066. Epub 2018 Mar 12
[PubMed PMID: 29594090]
[34]
Cooley CZ, Stockmann JP, Armstrong BD, Sarracanie M, Lev MH, Rosen MS, Wald LL. Two-dimensional imaging in a lightweight portable MRI scanner without gradient coils. Magnetic resonance in medicine. 2015 Feb:73(2):872-83. doi: 10.1002/mrm.25147. Epub 2014 Mar 25
[PubMed PMID: 24668520]
[35]
Abd-Ellah MK, Awad AI, Khalaf AAM, Hamed HFA. A review on brain tumor diagnosis from MRI images: Practical implications, key achievements, and lessons learned. Magnetic resonance imaging. 2019 Sep:61():300-318. doi: 10.1016/j.mri.2019.05.028. Epub 2019 Jun 5
[PubMed PMID: 31173851]
[36]
Booth TC, Wiegers EC, Warnert EAH, Schmainda KM, Riemer F, Nechifor RE, Keil VC, Hangel G, Figueiredo P, Álvarez-Torres MDM, Henriksen OM. High-Grade Glioma Treatment Response Monitoring Biomarkers: A Position Statement on the Evidence Supporting the Use of Advanced MRI Techniques in the Clinic, and the Latest Bench-to-Bedside Developments. Part 2: Spectroscopy, Chemical Exchange Saturation, Multiparametric Imaging, and Radiomics. Frontiers in oncology. 2021:11():811425. doi: 10.3389/fonc.2021.811425. Epub 2022 Feb 28
[PubMed PMID: 35340697]
[37]
Rodríguez-Porcel F, Hughes I, Anderson D, Lee J, Biller J. Foster Kennedy Syndrome Due to Meningioma Growth during Pregnancy. Frontiers in neurology. 2013:4():183. doi: 10.3389/fneur.2013.00183. Epub 2013 Nov 11
[PubMed PMID: 24273529]
[38]
Bouffet E, Jouvet A, Thiesse P, Sindou M. Chemotherapy for aggressive or anaplastic high grade oligodendrogliomas and oligoastrocytomas: better than a salvage treatment. British journal of neurosurgery. 1998 Jun:12(3):217-22
[PubMed PMID: 11013683]
[39]
Armstrong TS, Ying Y, Wu J, Acquaye AA, Vera-Bolanos E, Gilbert MR, Brown PD, Vardy J, Chung C. The relationship between corticosteroids and symptoms in patients with primary brain tumors: utility of the Dexamethasone Symptom Questionnaire-Chronic. Neuro-oncology. 2015 Aug:17(8):1114-20. doi: 10.1093/neuonc/nov054. Epub 2015 Apr 9
[PubMed PMID: 25862766]
[40]
Schrell UM, Rittig MG, Anders M, Kiesewetter F, Marschalek R, Koch UH, Fahlbusch R. Hydroxyurea for treatment of unresectable and recurrent meningiomas. I. Inhibition of primary human meningioma cells in culture and in meningioma transplants by induction of the apoptotic pathway. Journal of neurosurgery. 1997 May:86(5):845-52
[PubMed PMID: 9126901]
[41]
Liu B, Yu Y, Liu W, Deng T, Xiang D. Risk Factors for Non-arteritic Anterior Ischemic Optic Neuropathy: A Large Scale Meta-Analysis. Frontiers in medicine. 2021:8():618353. doi: 10.3389/fmed.2021.618353. Epub 2021 Oct 4
[PubMed PMID: 34671609]
Level 1 (high-level) evidence
[43]
Petramfar P, Hosseinzadeh F, Mohammadi SS. Pseudo-Foster Kennedy Syndrome as a Rare Presentation of Vitamin B12 Deficiency. Iranian Red Crescent medical journal. 2016 Jun:18(6):e24610. doi: 10.5812/ircmj.24610. Epub 2016 May 14
[PubMed PMID: 27621919]
[44]
Hussein MR, Baidas S. Advances in diagnosis and management of oligodendroglioma. Expert review of anticancer therapy. 2002 Oct:2(5):520-8
[PubMed PMID: 12382520]
Level 3 (low-level) evidence
[45]
Yeh SA, Lee TC, Chen HJ, Lui CC, Sun LM, Wang CJ, Huang EY. Treatment outcomes and prognostic factors of patients with supratentorial low-grade oligodendroglioma. International journal of radiation oncology, biology, physics. 2002 Dec 1:54(5):1405-9
[PubMed PMID: 12459363]
[46]
Vaquero J, Zurita M, Morales C, Coca S. Prognostic significance of tumor-enhancement and angiogenesis in oligodendroglioma. Acta neurologica Scandinavica. 2002 Jul:106(1):19-23
[PubMed PMID: 12067323]