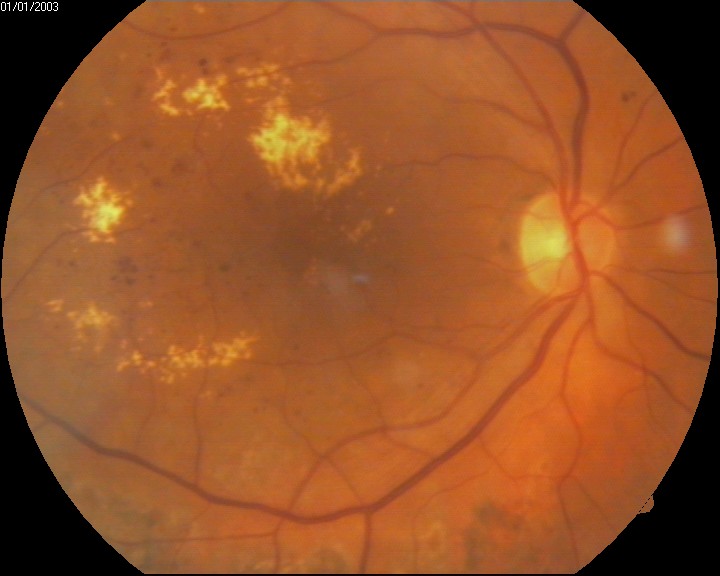
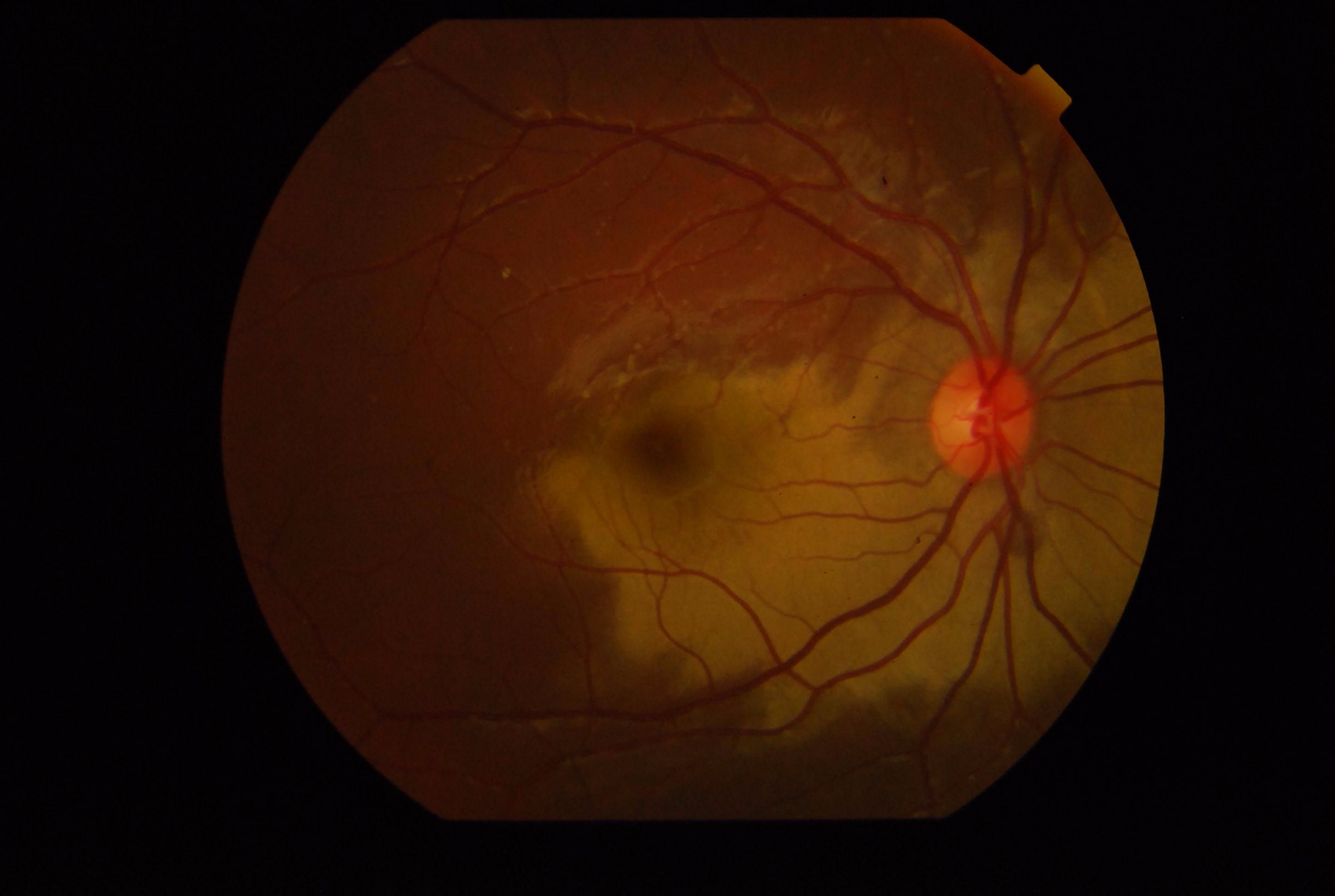
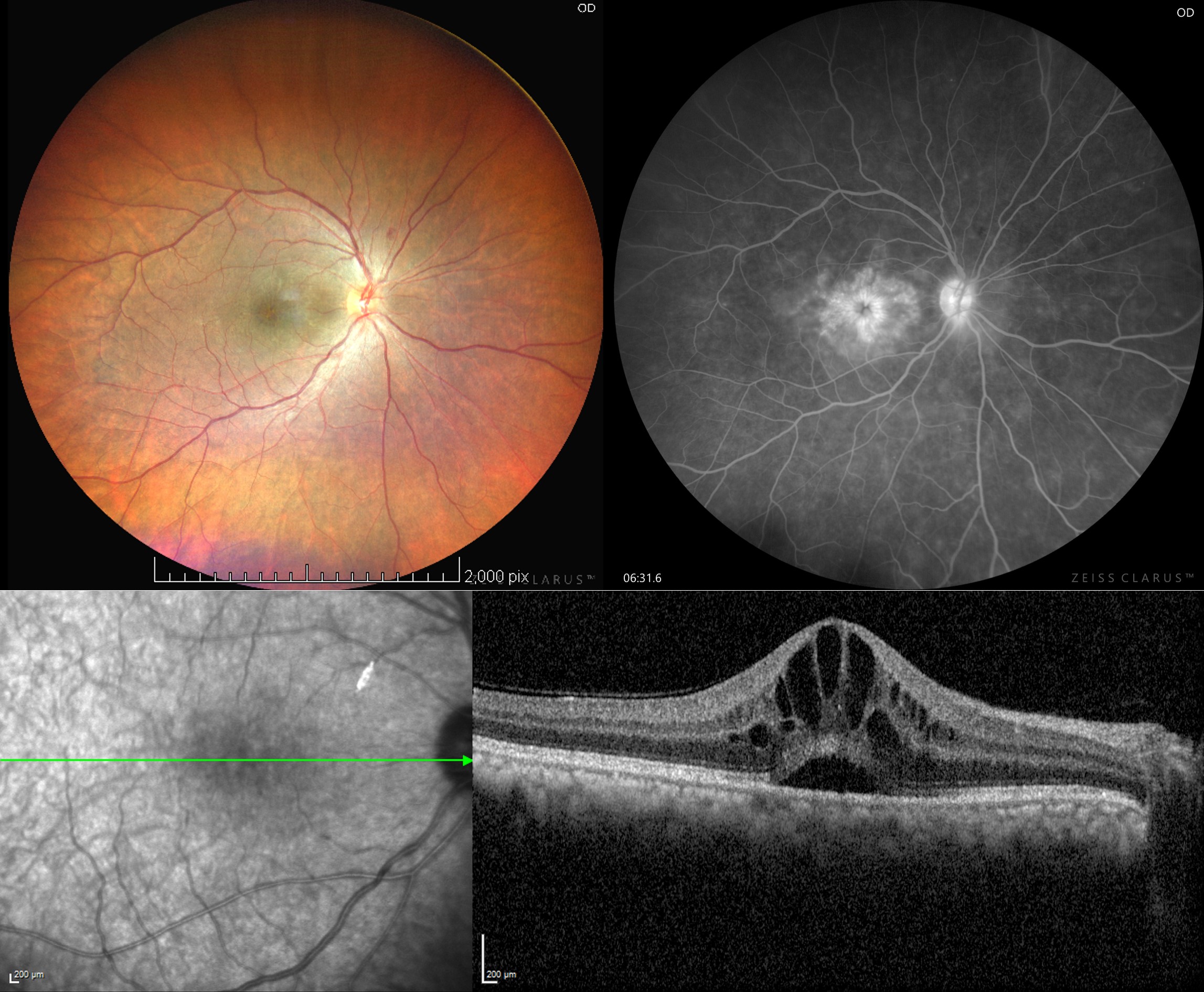
[1]
Tranos PG, Wickremasinghe SS, Stangos NT, Topouzis F, Tsinopoulos I, Pavesio CE. Macular edema. Survey of ophthalmology. 2004 Sep-Oct:49(5):470-90
[PubMed PMID: 15325193]
Level 3 (low-level) evidence
[2]
Rotsos TG, Moschos MM. Cystoid macular edema. Clinical ophthalmology (Auckland, N.Z.). 2008 Dec:2(4):919-30
[PubMed PMID: 19668445]
[3]
Coscas G, Cunha-Vaz J, Soubrane G. Macular edema: definition and basic concepts. Developments in ophthalmology. 2010:47():1-9. doi: 10.1159/000320070. Epub 2010 Aug 10
[PubMed PMID: 20703040]
[4]
Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, Rothschild PR, Omri S, Gélizé E, Jonet L, Delaunay K, De Kozak Y, Berdugo M, Zhao M, Crisanti P, Behar-Cohen F. Mechanisms of macular edema: Beyond the surface. Progress in retinal and eye research. 2018 Mar:63():20-68. doi: 10.1016/j.preteyeres.2017.10.006. Epub 2017 Nov 7
[PubMed PMID: 29126927]
[5]
Haydinger CD, Ferreira LB, Williams KA, Smith JR. Mechanisms of macular edema. Frontiers in medicine. 2023:10():1128811. doi: 10.3389/fmed.2023.1128811. Epub 2023 Mar 7
[PubMed PMID: 36960343]
[6]
Scholl S, Kirchhof J, Augustin AJ. Pathophysiology of macular edema. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2010:224 Suppl 1():8-15. doi: 10.1159/000315155. Epub 2010 Aug 18
[PubMed PMID: 20714176]
[7]
Cunha-Vaz J. Mechanisms of Retinal Fluid Accumulation and Blood-Retinal Barrier Breakdown. Developments in ophthalmology. 2017:58():11-20. doi: 10.1159/000455265. Epub 2017 Mar 28
[PubMed PMID: 28351041]
[9]
Venkatesh P, Chawla R, Tripathy K, Singh HI, Bypareddy R. Scleral resection in chronic central serous chorioretinopathy complicated by exudative retinal detachment. Eye and vision (London, England). 2016:3(1):23. doi: 10.1186/s40662-016-0055-5. Epub 2016 Sep 9
[PubMed PMID: 27617266]
[10]
Paulbuddhe V, Addya S, Gurnani B, Singh D, Tripathy K, Chawla R. Sympathetic Ophthalmia: Where Do We Currently Stand on Treatment Strategies? Clinical ophthalmology (Auckland, N.Z.). 2021:15():4201-4218. doi: 10.2147/OPTH.S289688. Epub 2021 Oct 20
[PubMed PMID: 34707340]
[11]
Tripathy K, Mittal K, Chawla R. Sympathetic ophthalmia following a conjunctival flap procedure for corneal perforation. BMJ case reports. 2016 Mar 14:2016():. doi: 10.1136/bcr-2016-214344. Epub 2016 Mar 14
[PubMed PMID: 26976837]
Level 3 (low-level) evidence
[12]
Chawla R, Kapoor M, Mehta A, Tripathy K, Vohra R, Venkatesh P. Sympathetic Ophthalmia: Experience from a Tertiary Care Center in Northern India. Journal of ophthalmic & vision research. 2018 Oct-Dec:13(4):439-446. doi: 10.4103/jovr.jovr_86_17. Epub
[PubMed PMID: 30479714]
[14]
Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye and vision (London, England). 2015:2():17. doi: 10.1186/s40662-015-0026-2. Epub 2015 Sep 30
[PubMed PMID: 26605370]
[15]
Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, Kowalski JW, Nguyen H, Wong TY, International Eye Disease Consortium. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010 Feb:117(2):313-9.e1. doi: 10.1016/j.ophtha.2009.07.017. Epub
[PubMed PMID: 20022117]
[16]
Laouri M, Chen E, Looman M, Gallagher M. The burden of disease of retinal vein occlusion: review of the literature. Eye (London, England). 2011 Aug:25(8):981-8. doi: 10.1038/eye.2011.92. Epub 2011 May 6
[PubMed PMID: 21546916]
[17]
Petrella RJ, Blouin J, Davies B, Barbeau M. Incidence and Characteristics of Patients with Visual Impairment due to Macular Edema Secondary to Retinal Vein Occlusion in a Representative Canadian Cohort. Journal of ophthalmology. 2012:2012():723169. doi: 10.1155/2012/723169. Epub 2012 Oct 14
[PubMed PMID: 23097691]
[18]
El Jammal T, Loria O, Jamilloux Y, Gerfaud-Valentin M, Kodjikian L, Sève P. Uveitis as an Open Window to Systemic Inflammatory Diseases. Journal of clinical medicine. 2021 Jan 14:10(2):. doi: 10.3390/jcm10020281. Epub 2021 Jan 14
[PubMed PMID: 33466638]
[19]
Henderson BA, Kim JY, Ament CS, Ferrufino-Ponce ZK, Grabowska A, Cremers SL. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. Journal of cataract and refractive surgery. 2007 Sep:33(9):1550-8
[PubMed PMID: 17720069]
[20]
Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Transactions of the American Ophthalmological Society. 1998:96():557-634
[PubMed PMID: 10360304]
[21]
Perente I, Utine CA, Ozturker C, Cakir M, Kaya V, Eren H, Kapran Z, Yilmaz OF. Evaluation of macular changes after uncomplicated phacoemulsification surgery by optical coherence tomography. Current eye research. 2007 Mar:32(3):241-7
[PubMed PMID: 17453944]
[22]
Koronis S, Stavrakas P, Balidis M, Kozeis N, Tranos PG. Update in treatment of uveitic macular edema. Drug design, development and therapy. 2019:13():667-680. doi: 10.2147/DDDT.S166092. Epub 2019 Feb 19
[PubMed PMID: 30858697]
[23]
Molaie AM, Pramil V, Hedges TR 3rd, Tomb LC, Vuong LN. Vitreoretinal Findings in Nonarteritic Ischemic Optic Neuropathy. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2022 Mar 1:42(1):e124-e129. doi: 10.1097/WNO.0000000000001264. Epub 2021 Apr 26
[PubMed PMID: 34001734]
[24]
Hedges TR 3rd, Vuong LN, Gonzalez-Garcia AO, Mendoza-Santiesteban CE, Amaro-Quierza ML. Subretinal fluid from anterior ischemic optic neuropathy demonstrated by optical coherence tomography. Archives of ophthalmology (Chicago, Ill. : 1960). 2008 Jun:126(6):812-5. doi: 10.1001/archopht.126.6.812. Epub
[PubMed PMID: 18541844]
[25]
Strong S, Liew G, Michaelides M. Retinitis pigmentosa-associated cystoid macular oedema: pathogenesis and avenues of intervention. The British journal of ophthalmology. 2017 Jan:101(1):31-37. doi: 10.1136/bjophthalmol-2016-309376. Epub 2016 Dec 2
[PubMed PMID: 27913439]
[26]
Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. European journal of ophthalmology. 2011:21 Suppl 6():S3-9. doi: 10.5301/EJO.2010.6049. Epub
[PubMed PMID: 23264323]
[27]
Owen LA, Hartnett ME. Soluble mediators of diabetic macular edema: the diagnostic role of aqueous VEGF and cytokine levels in diabetic macular edema. Current diabetes reports. 2013 Aug:13(4):476-80. doi: 10.1007/s11892-013-0382-z. Epub
[PubMed PMID: 23649946]
[28]
Ascaso FJ, Huerva V, Grzybowski A. The role of inflammation in the pathogenesis of macular edema secondary to retinal vascular diseases. Mediators of inflammation. 2014:2014():432685. doi: 10.1155/2014/432685. Epub 2014 Jul 22
[PubMed PMID: 25152567]
[29]
Toklu Y, Sarac O, Berk S, Simsek S. Angioedema after intravitreal bevacizumab injection. Cutaneous and ocular toxicology. 2012 Mar:31(1):85-6. doi: 10.3109/15569527.2011.609207. Epub
[PubMed PMID: 22309281]
[30]
Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, Waheed NK, Chakravarthy U, Rosenfeld PJ, Holz FG, Souied EH, Cohen SY, Querques G, Ohno-Matsui K, Boyer D, Gaudric A, Blodi B, Baumal CR, Li X, Coscas GJ, Brucker A, Singerman L, Luthert P, Schmitz-Valckenberg S, Schmidt-Erfurth U, Grossniklaus HE, Wilson DJ, Guymer R, Yannuzzi LA, Chew EY, Csaky K, Monés JM, Pauleikhoff D, Tadayoni R, Fujimoto J. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology. 2020 May:127(5):616-636. doi: 10.1016/j.ophtha.2019.11.004. Epub 2019 Nov 14
[PubMed PMID: 31864668]
Level 3 (low-level) evidence
[31]
Yeo NJY, Chan EJJ, Cheung C. Choroidal Neovascularization: Mechanisms of Endothelial Dysfunction. Frontiers in pharmacology. 2019:10():1363. doi: 10.3389/fphar.2019.01363. Epub 2019 Nov 29
[PubMed PMID: 31849644]
[32]
Gupta A, Paulbuddhe VS, Shukla UV, Tripathy K. Exudative Retinitis (Coats Disease). StatPearls. 2024 Jan:():
[PubMed PMID: 32809517]
[33]
Sen M, Shields CL, Honavar SG, Shields JA. Coats disease: An overview of classification, management and outcomes. Indian journal of ophthalmology. 2019 Jun:67(6):763-771. doi: 10.4103/ijo.IJO_841_19. Epub
[PubMed PMID: 31124484]
Level 3 (low-level) evidence
[34]
Pitkänen L, Tommila P, Kaarniranta K, Jääskeläinen JE, Kinnunen K. Retinal arterial macroaneurysms. Acta ophthalmologica. 2014 Mar:92(2):101-4. doi: 10.1111/aos.12210. Epub 2013 Jun 25
[PubMed PMID: 23800325]
[36]
García-O'Farrill N, Pugazhendhi S, Karth PA, Hunter AA. Radiation retinopathy intricacies and advances in management. Seminars in ophthalmology. 2022 May 19:37(4):417-435. doi: 10.1080/08820538.2021.2000623. Epub 2021 Dec 7
[PubMed PMID: 34874814]
Level 3 (low-level) evidence
[37]
Sahoo NK, Ranjan R, Tyagi M, Agrawal H, Reddy S. Radiation Retinopathy: Detection and Management Strategies. Clinical ophthalmology (Auckland, N.Z.). 2021:15():3797-3809. doi: 10.2147/OPTH.S219268. Epub 2021 Sep 8
[PubMed PMID: 34526764]
[38]
Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. International journal of radiation oncology, biology, physics. 1991 May 15:21(1):109-22
[PubMed PMID: 2032882]
[39]
Lambrecht M, Eekers DBP, Alapetite C, Burnet NG, Calugaru V, Coremans IEM, Fossati P, Høyer M, Langendijk JA, Méndez Romero A, Paulsen F, Perpar A, Renard L, de Ruysscher D, Timmermann B, Vitek P, Weber DC, van der Weide HL, Whitfield GA, Wiggenraad R, Roelofs E, Nyström PW, Troost EGC, work package 1 of the taskforce “European Particle Therapy Network” of ESTRO. Radiation dose constraints for organs at risk in neuro-oncology; the European Particle Therapy Network consensus. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2018 Jul:128(1):26-36. doi: 10.1016/j.radonc.2018.05.001. Epub 2018 May 17
[PubMed PMID: 29779919]
Level 3 (low-level) evidence
[40]
Brown GC, Shields JA, Sanborn G, Augsburger JJ, Savino PJ, Schatz NJ. Radiation retinopathy. Ophthalmology. 1982 Dec:89(12):1494-501
[PubMed PMID: 7162794]
[41]
Horgan N, Shields CL, Mashayekhi A, Shields JA. Classification and treatment of radiation maculopathy. Current opinion in ophthalmology. 2010 May:21(3):233-8. doi: 10.1097/ICU.0b013e3283386687. Epub
[PubMed PMID: 20393294]
Level 3 (low-level) evidence
[42]
Cimolai N. Comment on "Insights into the pathogenesis of cystoid macular edema: leukostasis and related cytokines". International journal of ophthalmology. 2020:13(8):1343-1344. doi: 10.18240/ijo.2020.08.25. Epub 2020 Aug 18
[PubMed PMID: 32821693]
Level 3 (low-level) evidence
[43]
Grzybowski A, Sikorski BL, Ascaso FJ, Huerva V. Pseudophakic cystoid macular edema: update 2016. Clinical interventions in aging. 2016:11():1221-1229
[PubMed PMID: 27672316]
[44]
Mahendradas P, Kawali A, Luthra S, Srinivasan S, Curi AL, Maheswari S, Ksiaa I, Khairallah M. Post-fever retinitis - Newer concepts. Indian journal of ophthalmology. 2020 Sep:68(9):1775-1786. doi: 10.4103/ijo.IJO_1352_20. Epub
[PubMed PMID: 32823394]
[46]
Tripathy K. Cystoid Macular Edema in Retinitis Pigmentosa with Intermediate Uveitis Responded Well to Oral and Posterior Subtenon Steroid. Seminars in ophthalmology. 2018:33(4):492-493. doi: 10.1080/08820538.2017.1303521. Epub 2017 Mar 29
[PubMed PMID: 28353369]
[47]
Tripathy K, Chawla R, Venkatesh P, Vohra R, Sharma YR, Gogia V, Jain S, Behera A. Ultra-wide Field Fluorescein Angiography in Retinitis Pigmentosa with Intermediate Uveitis. Journal of ophthalmic & vision research. 2016 Apr-Jun:11(2):237-9. doi: 10.4103/2008-322X.183929. Epub
[PubMed PMID: 27413510]
[49]
Mackool RJ, Muldoon T, Fortier A, Nelson D. Epinephrine-induced cystoid macular edema in aphakic eyes. Archives of ophthalmology (Chicago, Ill. : 1960). 1977 May:95(5):791-3
[PubMed PMID: 860942]
[50]
Drenser K, Sarraf D, Jain A, Small KW. Crystalline retinopathies. Survey of ophthalmology. 2006 Nov-Dec:51(6):535-49
[PubMed PMID: 17134644]
Level 3 (low-level) evidence
[52]
Domanico D, Verboschi F, Altimari S, Zompatori L, Vingolo EM. Ocular Effects of Niacin: A Review of the Literature. Medical hypothesis, discovery & innovation ophthalmology journal. 2015 Summer:4(2):64-71
[PubMed PMID: 26060832]
[54]
Chawla R, Tripathy K, Sharma A, Vohra R. Swept source optical coherence tomography-angiography of choroid in choroidal hemangioma before and after laser photocoagulation. Indian journal of ophthalmology. 2017 Aug:65(8):751-754. doi: 10.4103/ijo.IJO_974_16. Epub
[PubMed PMID: 28820167]
[55]
Garoon RB, Shields CL, Kaliki S, Shields JA. Cystoid macular edema as the initial manifestation of choroidal melanoma. Oman journal of ophthalmology. 2012 Sep:5(3):187-8. doi: 10.4103/0974-620X.106104. Epub
[PubMed PMID: 23440248]
[57]
Mansour AM, Elnahry AG, Tripathy K, Foster RE, Mehanna CJ, Vishal R, Çavdarlı C, Arrigo A, Parodi MB. Analysis of optical coherence angiography in cystoid macular oedema associated with gyrate atrophy. Eye (London, England). 2021 Jun:35(6):1766-1774. doi: 10.1038/s41433-020-01166-6. Epub 2020 Sep 1
[PubMed PMID: 32873946]
[58]
Tripathy K, Chawla R, Sharma YR, Gogia V. Ultrawide field fluorescein angiogram in a family with gyrate atrophy and foveoschisis. Oman journal of ophthalmology. 2016 May-Aug:9(2):104-6. doi: 10.4103/0974-620X.184529. Epub
[PubMed PMID: 27433038]
[59]
Jena S, Tripathy K, Chawla R, Mansour AM. Ultrawide field imaging to document the progression of gyrate atrophy of the choroid and retina over 5 years. BMJ case reports. 2021 Aug 17:14(8):. doi: 10.1136/bcr-2021-244695. Epub 2021 Aug 17
[PubMed PMID: 34404670]
Level 3 (low-level) evidence
[60]
Dave VP, Pappuru RR. An unusual presentation of nonarteritic ischemic optic neuropathy with subretinal fluid treated with intravitreal bevacizumab. Indian journal of ophthalmology. 2016 Jan:64(1):87-8. doi: 10.4103/0301-4738.178143. Epub
[PubMed PMID: 26953030]
[62]
Cunha-Vaz JG, Travassos A. Breakdown of the blood-retinal barriers and cystoid macular edema. Survey of ophthalmology. 1984 May:28 Suppl():485-92
[PubMed PMID: 6379947]
Level 3 (low-level) evidence
[63]
Wolter JR. The histopathology of cystoid macular edema. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. Albrecht von Graefe's archive for clinical and experimental ophthalmology. 1981:216(2):85-101
[PubMed PMID: 6910356]
[64]
Cusick M, Chew EY, Chan CC, Kruth HS, Murphy RP, Ferris FL 3rd. Histopathology and regression of retinal hard exudates in diabetic retinopathy after reduction of elevated serum lipid levels. Ophthalmology. 2003 Nov:110(11):2126-33
[PubMed PMID: 14597519]
[65]
Little K, Ma JH, Yang N, Chen M, Xu H. Myofibroblasts in macular fibrosis secondary to neovascular age-related macular degeneration - the potential sources and molecular cues for their recruitment and activation. EBioMedicine. 2018 Dec:38():283-291. doi: 10.1016/j.ebiom.2018.11.029. Epub 2018 Nov 22
[PubMed PMID: 30473378]
[67]
Martinez J, Smiddy WE, Kim J, Gass JD. Differentiating macular holes from macular pseudoholes. American journal of ophthalmology. 1994 Jun 15:117(6):762-7
[PubMed PMID: 8198160]
[68]
Sander B, Larsen M, Engler C, Strøm C, Moldow B, Larsen N, Lund-Andersen H. Diabetic macular oedema: a comparison of vitreous fluorometry, angiography, and retinopathy. The British journal of ophthalmology. 2002 Mar:86(3):316-20
[PubMed PMID: 11864891]
[69]
Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. The British journal of ophthalmology. 2004 Sep:88(9):1173-9
[PubMed PMID: 15317711]
[71]
Naseripour M, Hemmati S, Chaibakhsh S, Gordiz A, Miri L, Abdi F. Cystoid macular oedema without leakage in fluorescein angiography: a literature review. Eye (London, England). 2023 Jun:37(8):1519-1526. doi: 10.1038/s41433-022-02230-z. Epub 2022 Sep 10
[PubMed PMID: 36088420]
[72]
Prenner JL, Capone A Jr, Ciaccia S, Takada Y, Sieving PA, Trese MT. Congenital X-linked retinoschisis classification system. Retina (Philadelphia, Pa.). 2006 Sep:26(7 Suppl):S61-4
[PubMed PMID: 16946682]
[73]
Rao P, Dedania VS, Drenser KA. Congenital X-Linked Retinoschisis: An Updated Clinical Review. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2018 May-Jun:7(3):169-175. doi: 10.22608/APO.201803. Epub 2018 Apr 9
[PubMed PMID: 29633586]
[74]
Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The New England journal of medicine. 1993 Sep 30:329(14):977-86
[PubMed PMID: 8366922]
[75]
. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet (London, England). 1998 Sep 12:352(9131):837-53
[PubMed PMID: 9742976]
[76]
ACCORD Study Group, ACCORD Eye Study Group, Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC Jr, Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine LJ. Effects of medical therapies on retinopathy progression in type 2 diabetes. The New England journal of medicine. 2010 Jul 15:363(3):233-44. doi: 10.1056/NEJMoa1001288. Epub 2010 Jun 29
[PubMed PMID: 20587587]
[77]
Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d'Emden MC, Crimet DC, O'Connell RL, Colman PG, FIELD study investigators. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet (London, England). 2007 Nov 17:370(9600):1687-97
[PubMed PMID: 17988728]
Level 1 (high-level) evidence
[78]
Ng EW, Shima DT, Calias P, Cunningham ET Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nature reviews. Drug discovery. 2006 Feb:5(2):123-32
[PubMed PMID: 16518379]
[79]
Diabetic Retinopathy Clinical Research Network, Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, Friedman SM, Greven CM, Maturi RK, Pieramici DJ, Shami M, Singerman LJ, Stockdale CR. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007 Oct:114(10):1860-7
[PubMed PMID: 17698196]
Level 1 (high-level) evidence
[80]
. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Archives of ophthalmology (Chicago, Ill. : 1960). 1985 Dec:103(12):1796-806
[PubMed PMID: 2866759]
[81]
Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, Boos CJ, Xing W, Egan C, Peto T, Bunce C, Leslie RD, Hykin PG. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010 Jun:117(6):1078-1086.e2. doi: 10.1016/j.ophtha.2010.03.045. Epub 2010 Apr 22
[PubMed PMID: 20416952]
Level 1 (high-level) evidence
[82]
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A, RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011 Apr:118(4):615-25. doi: 10.1016/j.ophtha.2011.01.031. Epub
[PubMed PMID: 21459215]
[83]
Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS, RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012 Apr:119(4):789-801. doi: 10.1016/j.ophtha.2011.12.039. Epub 2012 Feb 11
[PubMed PMID: 22330964]
Level 1 (high-level) evidence
[84]
Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL 3rd, Friedman SM, Glassman AR, Scott IU, Stockdale CR, Sun JK, Diabetic Retinopathy Clinical Research Network. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011 Apr:118(4):609-14. doi: 10.1016/j.ophtha.2010.12.033. Epub
[PubMed PMID: 21459214]
Level 2 (mid-level) evidence
[85]
Do DV, Nguyen QD, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R, Berliner AJ, Gao B, Zeitz O, Ruckert R, Schmelter T, Sandbrink R, Heier JS, da Vinci Study Group. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012 Aug:119(8):1658-65. doi: 10.1016/j.ophtha.2012.02.010. Epub 2012 Apr 24
[PubMed PMID: 22537617]
[86]
Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, Heier JS, Terasaki H, Kaiser PK, Marcus DM, Nguyen QD, Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Zeitz O, Metzig C, Korobelnik JF. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology. 2015 Oct:122(10):2044-52. doi: 10.1016/j.ophtha.2015.06.017. Epub 2015 Jul 18
[PubMed PMID: 26198808]
[87]
Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, Melia M, Pieramici DJ, Sun JK, Beck RW. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. The New England journal of medicine. 2015 Mar 26:372(13):1193-203. doi: 10.1056/NEJMoa1414264. Epub 2015 Feb 18
[PubMed PMID: 25692915]
[88]
Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, Brucker AJ, Ferris FL, Hampton GR, Jhaveri C, Melia M, Beck RW, Diabetic Retinopathy Clinical Research Network. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. 2016 Jun:123(6):1351-9. doi: 10.1016/j.ophtha.2016.02.022. Epub 2016 Feb 27
[PubMed PMID: 26935357]
Level 2 (mid-level) evidence
[89]
Baker CW, Glassman AR, Beaulieu WT, Antoszyk AN, Browning DJ, Chalam KV, Grover S, Jampol LM, Jhaveri CD, Melia M, Stockdale CR, Martin DF, Sun JK, DRCR Retina Network. Effect of Initial Management With Aflibercept vs Laser Photocoagulation vs Observation on Vision Loss Among Patients With Diabetic Macular Edema Involving the Center of the Macula and Good Visual Acuity: A Randomized Clinical Trial. JAMA. 2019 May 21:321(19):1880-1894. doi: 10.1001/jama.2019.5790. Epub
[PubMed PMID: 31037289]
Level 1 (high-level) evidence
[90]
Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, Murahashi WY, Rubio RG, BRAVO Investigators. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010 Jun:117(6):1102-1112.e1. doi: 10.1016/j.ophtha.2010.02.021. Epub 2010 Apr 15
[PubMed PMID: 20398941]
[91]
Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, Rundle AC, Rubio RG, Murahashi WY, CRUISE Investigators. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010 Jun:117(6):1124-1133.e1. doi: 10.1016/j.ophtha.2010.02.022. Epub 2010 Apr 9
[PubMed PMID: 20381871]
[92]
Clark WL, Boyer DS, Heier JS, Brown DM, Haller JA, Vitti R, Kazmi H, Berliner AJ, Erickson K, Chu KW, Soo Y, Cheng Y, Campochiaro PA. Intravitreal Aflibercept for Macular Edema Following Branch Retinal Vein Occlusion: 52-Week Results of the VIBRANT Study. Ophthalmology. 2016 Feb:123(2):330-336. doi: 10.1016/j.ophtha.2015.09.035. Epub 2015 Oct 30
[PubMed PMID: 26522708]
[93]
Brown DM, Heier JS, Clark WL, Boyer DS, Vitti R, Berliner AJ, Zeitz O, Sandbrink R, Zhu X, Haller JA. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. American journal of ophthalmology. 2013 Mar:155(3):429-437.e7. doi: 10.1016/j.ajo.2012.09.026. Epub 2012 Dec 4
[PubMed PMID: 23218699]
[94]
Korobelnik JF, Holz FG, Roider J, Ogura Y, Simader C, Schmidt-Erfurth U, Lorenz K, Honda M, Vitti R, Berliner AJ, Hiemeyer F, Stemper B, Zeitz O, Sandbrink R, GALILEO Study Group. Intravitreal Aflibercept Injection for Macular Edema Resulting from Central Retinal Vein Occlusion: One-Year Results of the Phase 3 GALILEO Study. Ophthalmology. 2014 Jan:121(1):202-208. doi: 10.1016/j.ophtha.2013.08.012. Epub 2013 Sep 29
[PubMed PMID: 24084497]
[95]
Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, Gomes AV, Warburton J, Weichselberger A, Holz FG, HAWK and HARRIER Study Investigators. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2020 Jan:127(1):72-84. doi: 10.1016/j.ophtha.2019.04.017. Epub 2019 Apr 12
[PubMed PMID: 30986442]
Level 1 (high-level) evidence
[96]
Penha FM, Masud M, Khanani ZA, Thomas M, Fong RD, Smith K, Chand A, Khan M, Gahn G, Melo GB, Khanani AM. Review of real-world evidence of dual inhibition of VEGF-A and ANG-2 with faricimab in NAMD and DME. International journal of retina and vitreous. 2024 Jan 17:10(1):5. doi: 10.1186/s40942-024-00525-9. Epub 2024 Jan 17
[PubMed PMID: 38233896]
[97]
Taubenslag KJ, Kim SJ, Grzybowski A. Anti-inflammatory Pharmacotherapy for the Prevention of Cystoid Macular Edema After Cataract Surgery. American journal of ophthalmology. 2021 Dec:232():1-8. doi: 10.1016/j.ajo.2021.06.009. Epub 2021 Jun 19
[PubMed PMID: 34157275]
[98]
Friedman SM, Almukhtar TH, Baker CW, Glassman AR, Elman MJ, Bressler NM, Maker MP, Jampol LM, Melia M, Diabetic Retinopathy Clinical Research Network. Topical nepafenec in eyes with noncentral diabetic macular edema. Retina (Philadelphia, Pa.). 2015 May:35(5):944-56. doi: 10.1097/IAE.0000000000000403. Epub
[PubMed PMID: 25602634]
[99]
. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Early Treatment Diabetic Retinopathy Study Research Group. Archives of ophthalmology (Chicago, Ill. : 1960). 1995 Sep:113(9):1144-55
[PubMed PMID: 7661748]
[100]
Romero-Aroca P, Reyes-Torres J, Baget-Bernaldiz M, Blasco-Suñe C. Laser treatment for diabetic macular edema in the 21st century. Current diabetes reviews. 2014 Mar:10(2):100-12
[PubMed PMID: 24852439]
[101]
Writing Committee for the Diabetic Retinopathy Clinical Research Network, Fong DS, Strauber SF, Aiello LP, Beck RW, Callanan DG, Danis RP, Davis MD, Feman SS, Ferris F, Friedman SM, Garcia CA, Glassman AR, Han DP, Le D, Kollman C, Lauer AK, Recchia FM, Solomon SD. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Archives of ophthalmology (Chicago, Ill. : 1960). 2007 Apr:125(4):469-80
[PubMed PMID: 17420366]
[102]
Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008 Sep:115(9):1447-9, 1449.e1-10. doi: 10.1016/j.ophtha.2008.06.015. Epub 2008 Jul 26
[PubMed PMID: 18662829]
Level 1 (high-level) evidence
[103]
Miller SD. Argon laser photocoagulation for macular edema in branch vein occlusion. American journal of ophthalmology. 1985 Feb 15:99(2):218-9
[PubMed PMID: 4038586]
[104]
. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology. 1995 Oct:102(10):1425-33
[PubMed PMID: 9097788]
[105]
Pearson PA, Comstock TL, Ip M, Callanan D, Morse LS, Ashton P, Levy B, Mann ES, Eliott D. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology. 2011 Aug:118(8):1580-7. doi: 10.1016/j.ophtha.2011.02.048. Epub
[PubMed PMID: 21813090]
Level 1 (high-level) evidence
[106]
Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, Garretson B, Gupta A, Hariprasad SM, Bailey C, Reichel E, Soubrane G, Kapik B, Billman K, Kane FE, Green K, FAME Study Group. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012 Oct:119(10):2125-32. doi: 10.1016/j.ophtha.2012.04.030. Epub 2012 Jun 21
[PubMed PMID: 22727177]
[107]
Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, Chan CK, Gonzalez VH, Singerman LJ, Tolentino M, SCORE Study Research Group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Archives of ophthalmology (Chicago, Ill. : 1960). 2009 Sep:127(9):1115-28. doi: 10.1001/archophthalmol.2009.233. Epub
[PubMed PMID: 19752420]
Level 1 (high-level) evidence
[108]
Tripathy K. Commentary: Posterior subtenon triamcinolone - The unsung hero for managing various ocular disorders. Indian journal of ophthalmology. 2023 Jan:71(1):181-182. doi: 10.4103/ijo.IJO_1963_22. Epub
[PubMed PMID: 36588232]
Level 3 (low-level) evidence
[109]
Lavezzo MM, Sakata VM, Morita C, Rodriguez EE, Abdallah SF, da Silva FT, Hirata CE, Yamamoto JH. Vogt-Koyanagi-Harada disease: review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet journal of rare diseases. 2016 Mar 24:11():29. doi: 10.1186/s13023-016-0412-4. Epub 2016 Mar 24
[PubMed PMID: 27008848]
[110]
Boyer DS, Yoon YH, Belfort R Jr, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM, Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014 Oct:121(10):1904-14. doi: 10.1016/j.ophtha.2014.04.024. Epub 2014 Jun 4
[PubMed PMID: 24907062]
Level 1 (high-level) evidence
[111]
Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, Goodwin S, Aroney C, McAllister IL, Fraser-Bell S. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014 Dec:121(12):2473-81. doi: 10.1016/j.ophtha.2014.07.002. Epub 2014 Aug 22
[PubMed PMID: 25155371]
Level 1 (high-level) evidence
[112]
Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jacques ML, Jiao J, Li XY, Whitcup SM, OZURDEX GENEVA Study Group. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010 Jun:117(6):1134-1146.e3. doi: 10.1016/j.ophtha.2010.03.032. Epub 2010 Apr 24
[PubMed PMID: 20417567]
Level 1 (high-level) evidence
[113]
Maturi RK, Glassman AR, Liu D, Beck RW, Bhavsar AR, Bressler NM, Jampol LM, Melia M, Punjabi OS, Salehi-Had H, Sun JK, Diabetic Retinopathy Clinical Research Network. Effect of Adding Dexamethasone to Continued Ranibizumab Treatment in Patients With Persistent Diabetic Macular Edema: A DRCR Network Phase 2 Randomized Clinical Trial. JAMA ophthalmology. 2018 Jan 1:136(1):29-38. doi: 10.1001/jamaophthalmol.2017.4914. Epub
[PubMed PMID: 29127949]
Level 1 (high-level) evidence
[115]
Piccolino FC, De La Longrais RR, Manea M, Cicinelli S. Posterior cystoid retinal degeneration in central serous chorioretinopathy. Retina (Philadelphia, Pa.). 2008 Jul-Aug:28(7):1008-12. doi: 10.1097/IAE.0b013e31816b4b86. Epub
[PubMed PMID: 18698305]
[116]
Kessel L, Hamann S, Wegener M, Tong J, Fraser CL. Microcystic macular oedema in optic neuropathy: case series and literature review. Clinical & experimental ophthalmology. 2018 Dec:46(9):1075-1086. doi: 10.1111/ceo.13327. Epub 2018 Jun 20
[PubMed PMID: 29799159]
Level 2 (mid-level) evidence
[117]
Sipperley JO, Quigley HA, Gass DM. Traumatic retinopathy in primates. The explanation of commotio retinae. Archives of ophthalmology (Chicago, Ill. : 1960). 1978 Dec:96(12):2267-73
[PubMed PMID: 718521]
[118]
Kedarisetti KC, Narayanan R, Stewart MW, Reddy Gurram N, Khanani AM. Macular Telangiectasia Type 2: A Comprehensive Review. Clinical ophthalmology (Auckland, N.Z.). 2022:16():3297-3309. doi: 10.2147/OPTH.S373538. Epub 2022 Oct 10
[PubMed PMID: 36237488]
[120]
Sun JK, Lin MM, Lammer J, Prager S, Sarangi R, Silva PS, Aiello LP. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA ophthalmology. 2014 Nov:132(11):1309-16. doi: 10.1001/jamaophthalmol.2014.2350. Epub
[PubMed PMID: 25058813]
[121]
Uji A, Murakami T, Nishijima K, Akagi T, Horii T, Arakawa N, Muraoka Y, Ellabban AA, Yoshimura N. Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. American journal of ophthalmology. 2012 Apr:153(4):710-7, 717.e1. doi: 10.1016/j.ajo.2011.08.041. Epub 2011 Dec 3
[PubMed PMID: 22137207]
[122]
Liang MC, Vora RA, Duker JS, Reichel E. Solid-appearing retinal cysts in diabetic macular edema: a novel optical coherence tomography finding. Retinal cases & brief reports. 2013 Summer:7(3):255-8. doi: 10.1097/ICB.0b013e31828eef49. Epub
[PubMed PMID: 25391118]
Level 3 (low-level) evidence
[123]
Kashani AH, Lee SY, Moshfeghi A, Durbin MK, Puliafito CA. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY OF RETINAL VENOUS OCCLUSION. Retina (Philadelphia, Pa.). 2015 Nov:35(11):2323-31. doi: 10.1097/IAE.0000000000000811. Epub
[PubMed PMID: 26457395]
[124]
Forooghian F, Stetson PF, Meyer SA, Chew EY, Wong WT, Cukras C, Meyerle CB, Ferris FL 3rd. Relationship between photoreceptor outer segment length and visual acuity in diabetic macular edema. Retina (Philadelphia, Pa.). 2010 Jan:30(1):63-70. doi: 10.1097/IAE.0b013e3181bd2c5a. Epub
[PubMed PMID: 19952996]
[125]
Ota M, Nishijima K, Sakamoto A, Murakami T, Takayama K, Horii T, Yoshimura N. Optical coherence tomographic evaluation of foveal hard exudates in patients with diabetic maculopathy accompanying macular detachment. Ophthalmology. 2010 Oct:117(10):1996-2002. doi: 10.1016/j.ophtha.2010.06.019. Epub 2010 Aug 17
[PubMed PMID: 20723993]
[126]
Ranchod TM, Fine SL. Primary treatment of diabetic macular edema. Clinical interventions in aging. 2009:4():101-7
[PubMed PMID: 19503772]
[127]
Patel D, Patel SN, Chaudhary V, Garg SJ. Complications of intravitreal injections: 2022. Current opinion in ophthalmology. 2022 May 1:33(3):137-146. doi: 10.1097/ICU.0000000000000850. Epub 2022 Mar 9
[PubMed PMID: 35266893]
Level 3 (low-level) evidence
[128]
Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology. 2020 Jan:127(1):P66-P145. doi: 10.1016/j.ophtha.2019.09.025. Epub 2019 Sep 25
[PubMed PMID: 31757498]