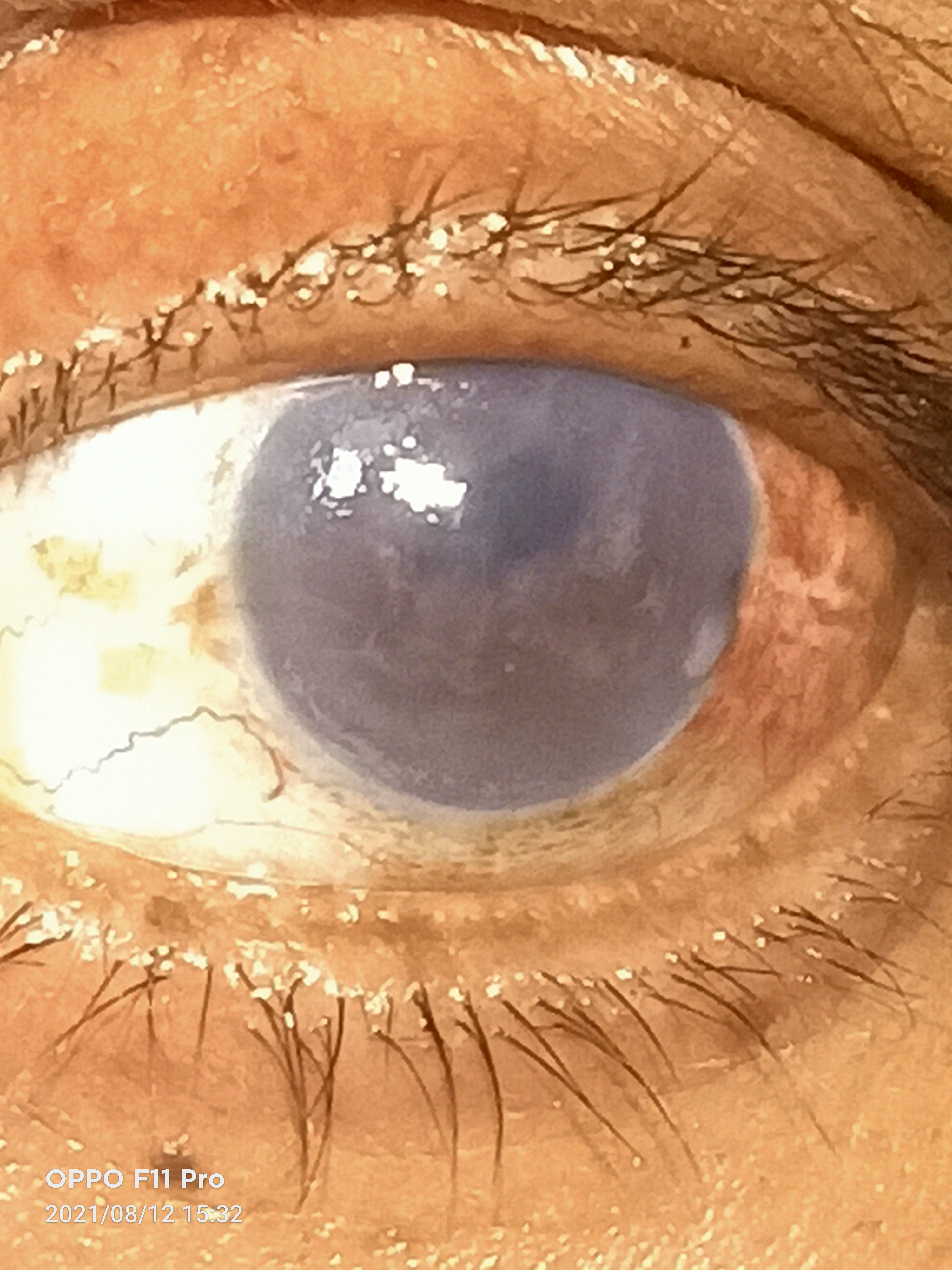
Continuing Education Activity
Pseudophakic bullous keratopathy or postoperative corneal edema is characterized by epithelial and subepithelial bullae, stromal edema, and endothelial decompensation secondary to trauma inflicted during cataract surgery especially post phacoemulsification. The patient presents with pain, watering, redness, and gradually worsening visual acuity over months. The conservative management is characterized by topical hypertonic agents, cycloplegics, and antiglaucoma drugs. The gold standard treatment is corneal transplantation. This activity highlights the etiology, epidemiology, pathophysiology, clinical presentation, investigations, diagnostic modalities, treatment options, differential diagnosis, and complications of pseudophakic bullous keratopathy.
Objectives:
- Describe the risk factors for developing pseudophakic bullous keratopathy.
- Summarize the evaluation of pseudophakic bullous keratopathy.
- Explain the management of pseudophakic bullous keratopathy.
- Outline the potential complications of pseudophakic bullous keratopathy.
Introduction
Pseudophakic bullous keratopathy (PBK) is characterized by the development of irreversible corneal edema after cataract surgery. The initial insult occurs at the corneal endothelium, following which there is progressive stromal edema. This edema can progress to the subepithelial and epithelial layers and results in bullae formation, hence the name bullous keratopathy.[1] Trauma to endothelium can occur during any intraocular surgery, but PBK is most commonly reported after phacoemulsification.[2]
PBK has also been reported after excessive and improper use of ultrasound energy during phacoemulsification, complicated cataract surgery, anterior vitrectomy close to the endothelium, nucleus rub to the endothelium while nucleus delivery in manual small incision cataract surgery, Fuch’s endothelial dystrophy, and other endothelial dystrophy cases.[3] As surgical techniques have evolved and newer IOL designs have come into the market, the incidence of PBK has subsided to a good extent. However, it remains an important cause of visual morbidity following a routine and complicated cataract surgical procedure.
Etiology
The main etiology of PBK is due to endothelial loss post-surgical trauma, especially in older patients. The other main causes are endothelial burns secondary high energy use during phacoemulsification. High irrigation and aspiration can also result in endothelial damage during cataract surgery and manifest as striate keratopathy post-cataract surgery. The other etiologies linked with PBK are all diseases affecting corneal endothelium like:
- Fuchs endothelial dystrophy
- Posterior polymorphous endothelial dystrophy
- Congenital hereditary endothelial dystrophy[4]
- Iridocorneal endothelial (ICE) syndrome-Chandler syndrome
- Essential/progressive iris atrophy
- Iris nevus / Cogan-Reese syndrome[5]
- Herpetic disciform keratitis[6]
- Diabetes mellitus[7]
- Pseudoexfoliation syndrome[8]
- Narrow-angle glaucoma[9]
- Blunt trauma[10][11]
- Endothelitis, uveitis, keratitis[12]
- Surgical trauma during any intraocular surgery[13]
- Toxic anterior segment syndrome (TASS)[14]
- Silicon oil in the anterior chamber[15]
- Implantable collamer lens[16]
- Vitreous touch syndrome[17]
- Vitreous wick syndrome[18]
The intraoperative etiology is linked to closed-loop anterior chamber intraocular lens (ACIOL) > open-loop ACIOL> iris fixated IOL> posterior chamber IOL.[3] Postoperative etiologies include after routine surgery there is usually 10 % endothelial cell loss, 1% endothelial loss/ year naturally, vitreous touch syndrome, vitreous wick syndrome, flat anterior chamber post-surgery Intermittent IOL touch.[19][20]
Epidemiology
Although its difficult to estimate the exact incidence of PBK, but the current reported rate of PBK in the USA is 0.1%. During the years 1978 to 1982, various US FDA-approved studies showed the incidence of 0.06% of PBK after PCIOL implantation, 1.2% after anterior chamber lenses and 1.5% after iris fixated lenses.[21]
The long-term reported incidence of PBK is 15%. The amount of endothelial cell loss during cataract surgery can also be correlated to PBK, which is estimated to be approximately 4.72% after extracapsular cataract extraction (ECCE), 4.21% after small incision cataract surgery (SICS), and 5.41% after phacoemulsification. There is no gender, sex, or racial predilection for PBK. The most common risk factor for PBK is Fuchs dystrophy which is 3 times more common in females. Old age is another important risk factor as the endothelial count reduces with age.[22]
Pathophysiology
The ability of the cornea to remain in a dehydrated state determines its transparency. The epithelium and endothelium act as a barrier to water and electrolytes due to their semipermeable membrane property. The NaKATPase pump located in the lateral cell membrane of the endothelium prevents corneal hydration. The endothelial cells pump the fluid out of the corneal stroma and help in maintaining transparency.
Any damage to endothelial cells will hamper corneal transparency when aqueous entering the cornea cannot be pumped out due to fewer viable endothelial cells. Approximately 700 cells/ mm square are required to maintain transparency. The endothelium lacks mitotic activity. At birth, the endothelial cell count is 7500 cells/mm^2, and it reduces to 2500 to 2700 cells/mm^2 by adulthood. The average cell loss per year is 0.5% after the age of 20. Surgically induced trauma, persistent inflammation, hereditary conditions like Fuch’s, and congenital hereditary endothelial dystrophy can promote this cell loss.
When the cell density reaches around 300-500 cells/ mm^2, it is called critically low cell density, resulting in the development of PBK. It results in increased polymegathism (irregular small and large cells) and pleomorphism (abnormal cell shape, increase non-hexagonality). There is abnormal production of the Descemet membrane; the pump fails and manifests as stromal edema.
The corneal anterior stromal has a high ratio of dermatan to keratan sulfate, making the posterior stroma swell more whenever there is an endothelial failure. This edema can change in response to a change in intraocular pressure (IOP). Imbibition pressure = IOP - swelling pressure. The aqueous can migrate from stroma to epithelium resulting in epithelial edema, blisters, and bullae formation. Early morning edema is seen due to lack of evaporation and hypertonic environment overnight.[23]
Histopathology
The various histopathological features in PBK are loss of corneal endothelial cells, pleomorphism and polymegathism of endothelial cells, reduced stromal keratocytes, thickening of posterior collagen layer of Descemet membrane, lack of adhesive proteins like laminin, fibronectin, and type 4 collagen in the epithelial basement membrane, increased antiadhesive proteins like tenascin C and thrombospondin-1 and increases levels of interleukin 8 (IL-8), interleukin 2 (IL-2), insulin-like growth factor (IGF-1), bone marrow factor-4 (BMP-4), and transforming growth factor (TGF- β).[24]
History and Physical
The clinical diagnosis of PBK is made by detailed history and clinical evaluation.
History
The patient usually gives a history of gradually progressive vision loss after cataract surgery. The patient may also give a history of prolonged surgical time or a history of intraocular complications. The patient also gives a history of early morning blurring due to overnight fluid accumulation and corneal edema.
Symptoms
At presentation, the patient may be asymptomatic. In the early stages, the patient may complain of blurring of vision. In advanced stages, the patient will complain of severe loss of vision. Other symptoms can be pain, redness, excessive photophobia, irritation, foreign body sensation, and halos around light.
Signs
A meticulously performed slit-lamp examination may reveal findings in each layer of the cornea. In the epithelium, there can be an epithelial defect, epithelial edema, microcysts, and bullae. The subepithelial layer may show scarring or haze formation, the stromal layer shows stromal edema, scarring, and haze, the Descemet membrane shows folds and scarring, and the endothelium reveals edema and decompensation.
Other signs to look for are the type of surgical incision (superior scleral incision for SICS and superior or temporal clear corneal for phacoemulsification), corneal neovascularization, shallow anterior chamber, iris chaffing, atrophic patches, iridodialysis, peripheral anterior synechia or posterior synechia, peripheral iridectomy pupil irregularity, pupil peaking, posterior capsular rupture, zonular dialysis, the position of IOL whether anterior, iris fixated, iris-claw, sulcus or posterior chamber IOL, optic capture and any vitreous prolapse in the anterior chamber, vitreous touch syndrome or vitreous wick syndrome. The other eye should also be examined for guttae and to rule out Fuch’s endothelial, congenital hereditary endothelial dystrophy, posterior polymorphous endothelial dystrophy, or any other endothelial pathology. The guttae appear as a golden brown confluent excrescence of Descemet membrane and give endothelium a beaten bronze appearance.[3]
Evaluation
Imaging Studies
Specular Microscopy
It basically means photographic mapping of the corneal endothelium. When light rays fall onto the cornea, the reflected images from the optical interface can be imaged (endothelium or aqueous humor). The endothelial layer is mapped under high magnification, and thus the cell density can be quantified. The normal cell density is 3000 to 3500 cell/mm^2 in young individuals and 2000 to 2500 cell/mm^2 in older adults. A cell density below 1000 cells/ mm^2 is labeled as critical cell density, and individuals with such cell density are at the highest risk of developing corneal decompensation. The various endothelial parameters assessed are polymorphism (variation in cell shape), polymegathism (variation in cell size), the maximum or minimum number of cells, average cells, number of cells showing hexagonality, coefficient of variation, standard deviation, presence or absence of guttae and artifacts. Endothelial cells depicting various sizes and shapes are considered under stress and are abnormal.[25]
Ultrasound Pachymetry
The corneal thickness can be easily assessed using ultrasound and corneal pachymetry. The normal central corneal thickness is 0.55 mm, and it increases to 0.80 in the corneal periphery. In conditions causing cornea thickening and edema, the thickness is usually more than 0.60, indicating corneal edema. The clinical application for pachymetry is to help assess the progression of endothelial diseases like Fuch’s endothelial dystrophy and follow up of corneal graft rejection cases under steroid regimen.[26]
Optical Pachymetry
This imaging modality helps assess the depth of corneal pathology (e.g., nebular, macular, or leucomatous corneal opacities), especially when full-thickness stroma is not involved, and it becomes necessary to decide for depth before preoperative excimer laser phototherapeutic keratectomy.[27]
Treatment / Management
Medical Management
Hyperosmotic Agents
Topical sodium chloride- 2% drops and 5% ointment. These agents help by forming a hypertonic tear film by imbibing water out of the cornea. The drops are usually prescribed 4 times per day and ointment at bedtime to reduce early morning corneal edema due to accumulation of fluid overnight.[28]
Antiglaucoma Drugs
The drugs commonly employed are beta-blockers and alpha agonists—these work by reducing the intraocular pressure, which reduces corneal edema and thickness in the postoperative period. Miotics and prostaglandins are avoided because they usually aggravate inflammation. Carbonic anhydrase inhibitors are also avoided as they are epitheliotoxic.[29]
Steroids
Steroids are usually employed to reduce acute inflammation or postoperative uveitis immediately after cataract surgery. Before starting steroids, the cornea must always be stained with fluorescein to rule out any epithelial defect or infectious keratitis.[30]
Lubricants
They are used to lubricate the ocular surface and cornea to prevent surface irritation and dryness.[31]
Contact Lens
Extended wear of soft contact lenses plays a huge role in the management of patients with PBK. The various added advantages of contact lens are that they alleviate pain associate with epithelial bullae, protect infectious keratitis again when bullae are ruptured, and there is an epithelial defect, help to improve visual acuity when used in conjunction with hypertonic saline, and also prevents the formation of new bullae due to its barrier effect.[32][33][34]
Surgical Management
Gunderson Conjunctival Flap
In this technique, a thin flap of the conjunctiva, usually from the superior bulbar conjunctiva, is transposed over the cornea with intact nasal and temporal bridges. It meets the metabolic demands of the cornea, increases local blood supply, helps in healing, and replaces the dead and denuded epithelium[35]
Amniotic Membrane Grafting (AMG)
In this technique, first, the loose epithelium is removed with the help of a sponge or a blade. This results in a large epithelial defect, usually sparing the limbus. A required amniotic membrane is cut and placed over the cornea with the stromal side towards the cornea, which is sticky compared to the basement membrane. The AMG is then attached to the cornea with the help of interrupted stay sutures. If needed, a BCL can also be placed.[36]
Bowman’s Membrane Cautery
This procedure helps in pain relief by forming a tight barrier between epithelium and stroma. This prevents fluid migration to the epithelium and prevents bullae formation[37]
Anterior Stromal Puncture
In this technique, multiple stromal punctures are created with the help of a 26G needle sparing the corneal periphery. This helps in pain and symptomatic relief. It results in subepithelial scarring and fibrosis in all cases.[38]
Annular Keratotomy
This technique helps in pain relief in symptomatic patients without visual potential. A partial-thickness trephination of the cornea is performed in an annular fashion. This helps in pain relief by severing the branches corneal ciliary nerve and reduce corneal sensations.[39]
Basement Membrane Polishing
It is done with the help of a diamond burr. After removing the epithelium, a 4.5 to 5 mm diamond burr can be used to gently polish the basement membrane across the area of epithelial debridement.[40]
Phototherapeutic Keratectomy (PTK)
The laser used for PTK is Nidek EC 500 excimer laser. This is of three types:
- Superficial PTK- Ablation of 8 to 25 um stroma with 62% success rate
- Intermediate PTK- Ablation of 50 to 100 um stroma with 42% success rate
- Deep PTK- Ablation of 25% stroma with 60% success rate[41]
Thoman et al., in their analysis, proposed that superficial PTK is an effective procedure for pain relief and bullae resolution in the majority of the patients. They also highlighted that deep ablation has a better effect in reducing the pain by ablation of the preterminal neural plexus of the cornea located deep to the bowman’s membrane. It also reduces stromal edema by reducing the quantity of mucopolysaccharide and results in increased scarring with improved stability of the epithelium.[42]
Corneal Collagen Crosslinking with Riboflavin (C3R)
C3R is a new treatment tool for the temporary reduction of corneal edema in patients with PBK. The free radical generated by the action of riboflavin with UVA light induces cross-linking of stroma and strengthens the bonds. It has been found useful to reduce edema, thickness, improve corneal transparency and alleviate pain after surgery.[43]
Corneal Transplantation
Penetrating Keratoplasty
This is the gold standard surgery. In this technique, full-thickness host cornea is replaced by a donor cornea which is opposed to the host cornea with interrupted or continuous running sutures. The visual recovery takes some time. The graft size is usually 7 to 7.5 mm to avoid complications of small and large grafts, such as astigmatism and secondary glaucoma.
Descemet Stripping Endothelial Keratoplasty (DSEK) or Descemet Stripping Automated Endothelial Keratoplasty (DSAEK)
DSAEK was first introduced and popularised by Melles and colleagues, which has revolutionized PBK management off late. In this technique, the Descemet membrane is stripped off from the host and replaced with donor Descemet membrane along with some part of the posterior stroma. Then an air bubble is introduced for 20 minutes to ensure a perfect opposition. Before completing the surgery, 10 to 20 percent of the air bubble is removed to prevent postoperative pupillary block glaucoma. If the donor is prepared manually, it is called DSEK, and if the automated anterior chamber is employed, it is called DSAEK.[44]
Descemet Membrane Endothelial Keratoplasty (DMEK)
This technique is a further refinement over DSEK. In this, the Descemet and endothelium of the host are replaced with the endothelium without any stromal tissue. Hence, this technique offers quicker visual recovery, better corrected distant visual acuity, and reduced risk of graft rejection. DMEK has a steep learning curve and requires more skill and expertise to get the desired outcome
Hence, in a nutshell, endothelial keratoplasty offers a lot of advantages over traditional penetrating keratoplasty. The few advantages are reduced risk of graft rejection, being a closed globe procedure, there is minimal chance of expulsive choroidal hemorrhage, the suture related complications are avoided, the corneal contour and topography are preserved, there is less induced astigmatism, there is no need for long term steroid use, and more wound strength due to smaller incision. The various disadvantages are interphase haze, steep learning curve, and graft/ lenticule dislocation, which may require rebubbling.[45][46]
Differential Diagnosis
- Fuch’s endothelial dystrophy
- Congenital hereditary endothelial dystrophy (CHED)
- Posterior polymorphous corneal dystrophy (PPMD)
- Iridocorneal endothelial syndrome (ICE)
- Herpetic stromal keratitis (HSK)
- Recurrent corneal erosion syndrome (RCES)
Prognosis
Timely and meticulously done optical penetrating keratoplasty or Descemet stripping endothelial keratoplasty (DSEK) has a good prognosis in pseudophakic bullous keratopathy cases. However, the medical treatment is for symptomatic relief, and cases treated with medical treatment alone usually have a poor prognosis.
Complications
Medical
- Irreversible corneal edema
- Dry eyes
- Drug toxicity
Surgical
- Graft failure
- Iatrogenic trauma
- Graft infection
- Secondary glaucoma
- Expulsive choroidal hemorrhage
- Vitreous prolapse
- Cystoid macular edema[3]
Postoperative and Rehabilitation Care
Good postoperative care is extremely vital in these cases for graft survival and visual rehabilitation. Post keratoplasty, the patient should be started on 2 hourly topical 1% prednisolone or 0.1% dexamethasone for 3 days, followed by 6 times for 15 days and then 4, 3, 2, and 1 time for 3 months each and later once night time maintenance dose for graft survival. In addition, the adjuvant drugs in the form of 2% homatropine two times for 15 days, topical 0.5% timolol two times along with steroids to prevent secondary glaucoma, and oral anti-inflammatory for pain relief. The patient should be meticulously counseled regarding the importance of regular use of medication and timely follow-up. The patient must also be counseled to report immediately if there is pain, redness and, sudden onset blurred vision to prevent graft rejection. The patient must also be counseled that good visual acuity returns by around 3 to 4 months.
Consultations
Any patient presenting to the outpatient department with pain, photophobia, redness, and early morning blurred vision after cataract surgery should be examined carefully by an ophthalmologist to rule out pseudophakic bullous keratopathy. This patient must be referred to a cornea and ocular surface expert for a higher opinion. The cornea specialist is an expert in picking up shallow Descemet membrane detachment (DMD) that may lead to corneal decompensation in the future. They are highly skilled in deciding whether the patient needs DM to reposition with an air bubble or whether the patient needs corneal transplantation in the form of PKP or DSEK.
Deterrence and Patient Education
Patient education plays a key role in managing the cases of PBK well. The patient should be educated in detail regarding the ocular pathology and mechanism behind corneal edema. The patient should be educated regarding the management options, and they may require a transplant later on. The other eye should be scrutinized to rule out pre-existing endothelial pathology, and the patient must be educated regarding the same. The patient should also be educated regarding the hygiene and maintenance of the bandage contact lens if required after bullae rupture. Patients must be educated regarding the importance of regular and timely use of steroids and meticulous follow-up. He must be educated regarding steroid taper to prevent complications and irreversible visual sequelae.
Pearls and Other Issues
To summarize, PBK is a common ocular pathology encountered in day-to-day clinical practice after routine or complicated cataract surgery. With rapid advancements in surgical technique, better training of young ophthalmologists, reduce implantation of ACIOL and IFIOL in complicated cases, and improvement in IOL designs, the incidence of PBK has reduced drastically. But, it remains one of the common causes of corneal transplantation across the globe. Prompt diagnosis and meticulous management in expert hands can safeguard visual acuity in the majority of these cases.
Enhancing Healthcare Team Outcomes
Any case of PBK presenting to the OPD must be evaluated carefully. A detailed history and meticulous slit lamp evaluation are mandated by a cornea expert to guide the management. The nursing team and the paramedical staff have a huge role in preoperative workup and counseling, assistance during corneal transplantation, intraoperative and postoperative patient management. A patient-centered approach definitely improves outcomes in these cases. In addition, the nursing team and pharmacists must also counsel the patient regarding the complications of surgery and side effects of irregular use of drugs to ensure safety and excellent outcome in these cases.