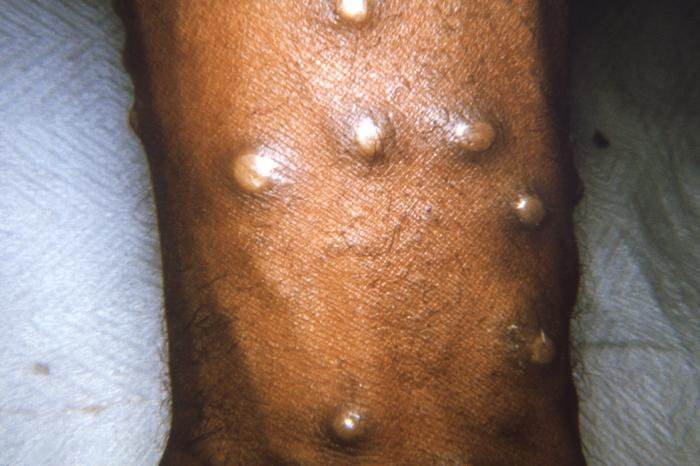
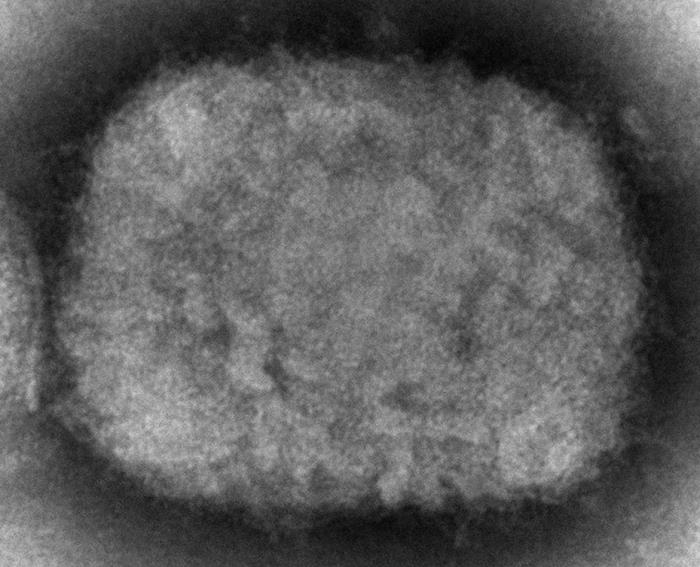
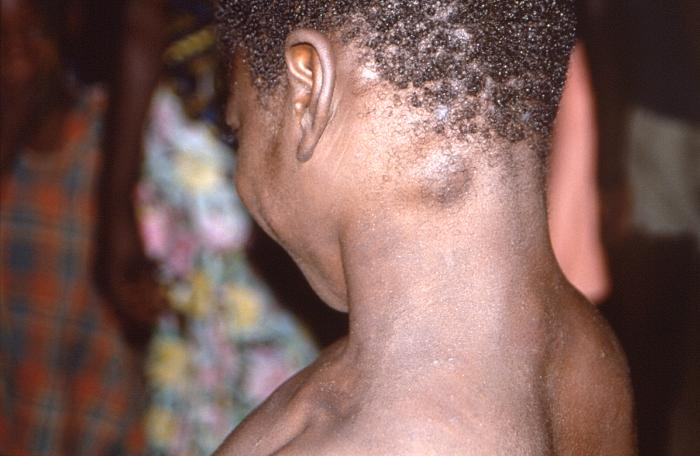
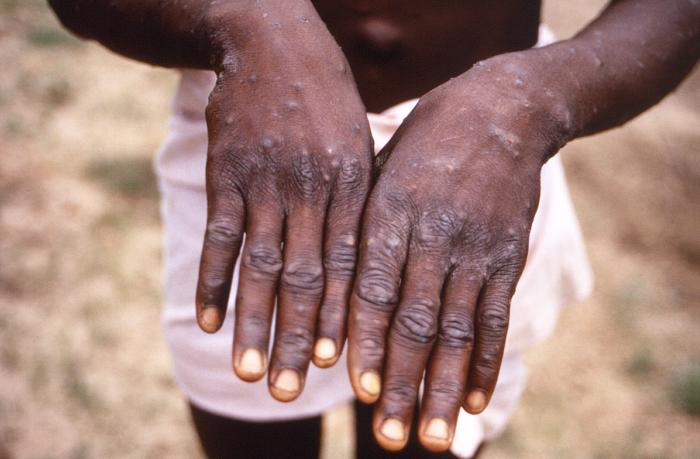
[2]
Ladnyj ID,Ziegler P,Kima E, A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bulletin of the World Health Organization. 1972;
[PubMed PMID: 4340218]
[3]
Nguyen PY,Ajisegiri WS,Costantino V,Chughtai AA,MacIntyre CR, Reemergence of Human Monkeypox and Declining Population Immunity in the Context of Urbanization, Nigeria, 2017-2020. Emerging infectious diseases. 2021 Apr;
[PubMed PMID: 33756100]
[4]
Sklenovská N,Van Ranst M, Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Frontiers in public health. 2018;
[PubMed PMID: 30234087]
[5]
Alakunle E,Moens U,Nchinda G,Okeke MI, Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses. 2020 Nov 5;
[PubMed PMID: 33167496]
[6]
Kugelman JR,Johnston SC,Mulembakani PM,Kisalu N,Lee MS,Koroleva G,McCarthy SE,Gestole MC,Wolfe ND,Fair JN,Schneider BS,Wright LL,Huggins J,Whitehouse CA,Wemakoy EO,Muyembe-Tamfum JJ,Hensley LE,Palacios GF,Rimoin AW, Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerging infectious diseases. 2014 Feb;
[PubMed PMID: 24457084]
[7]
Walsh D, Poxviruses: Slipping and sliding through transcription and translation. PLoS pathogens. 2017 Nov;
[PubMed PMID: 29145493]
[8]
Peiró-Mestres A,Fuertes I,Camprubí-Ferrer D,Marcos MÁ,Vilella A,Navarro M,Rodriguez-Elena L,Riera J,Català A,Martínez MJ,Blanco JL,Hospital Clinic de Barcelona Monkeypox Study Group., Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2022 Jul
[PubMed PMID: 35837964]
[10]
Centers for Disease Control and Prevention (CDC)., Multistate outbreak of monkeypox--Illinois, Indiana, and Wisconsin, 2003. MMWR. Morbidity and mortality weekly report. 2003 Jun 13;
[PubMed PMID: 12803191]
[11]
Erez N,Achdout H,Milrot E,Schwartz Y,Wiener-Well Y,Paran N,Politi B,Tamir H,Israely T,Weiss S,Beth-Din A,Shifman O,Israeli O,Yitzhaki S,Shapira SC,Melamed S,Schwartz E, Diagnosis of Imported Monkeypox, Israel, 2018. Emerging infectious diseases. 2019 May;
[PubMed PMID: 30848724]
[12]
Yong SEF,Ng OT,Ho ZJM,Mak TM,Marimuthu K,Vasoo S,Yeo TW,Ng YK,Cui L,Ferdous Z,Chia PY,Aw BJW,Manauis CM,Low CKK,Chan G,Peh X,Lim PL,Chow LPA,Chan M,Lee VJM,Lin RTP,Heng MKD,Leo YS, Imported Monkeypox, Singapore. Emerging infectious diseases. 2020 Aug;
[PubMed PMID: 32338590]
[13]
Hobson G,Adamson J,Adler H,Firth R,Gould S,Houlihan C,Johnson C,Porter D,Rampling T,Ratcliffe L,Russell K,Shankar AG,Wingfield T, Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2021 Aug;
[PubMed PMID: 34387184]
Level 3 (low-level) evidence
[14]
Rao AK,Schulte J,Chen TH,Hughes CM,Davidson W,Neff JM,Markarian M,Delea KC,Wada S,Liddell A,Alexander S,Sunshine B,Huang P,Honza HT,Rey A,Monroe B,Doty J,Christensen B,Delaney L,Massey J,Waltenburg M,Schrodt CA,Kuhar D,Satheshkumar PS,Kondas A,Li Y,Wilkins K,Sage KM,Yu Y,Yu P,Feldpausch A,McQuiston J,Damon IK,McCollum AM,July 2021 Monkeypox Response Team., Monkeypox in a Traveler Returning from Nigeria - Dallas, Texas, July 2021. MMWR. Morbidity and mortality weekly report. 2022 Apr 8;
[PubMed PMID: 35389974]
[15]
Costello V,Sowash M,Gaur A,Cardis M,Pasieka H,Wortmann G,Ramdeen S, Imported Monkeypox from International Traveler, Maryland, USA, 2021. Emerging infectious diseases. 2022 May;
[PubMed PMID: 35263559]
[16]
Rimoin AW,Mulembakani PM,Johnston SC,Lloyd Smith JO,Kisalu NK,Kinkela TL,Blumberg S,Thomassen HA,Pike BL,Fair JN,Wolfe ND,Shongo RL,Graham BS,Formenty P,Okitolonda E,Hensley LE,Meyer H,Wright LL,Muyembe JJ, Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proceedings of the National Academy of Sciences of the United States of America. 2010 Sep 14;
[PubMed PMID: 20805472]
[17]
Petersen BW,Kabamba J,McCollum AM,Lushima RS,Wemakoy EO,Muyembe Tamfum JJ,Nguete B,Hughes CM,Monroe BP,Reynolds MG, Vaccinating against monkeypox in the Democratic Republic of the Congo. Antiviral research. 2019 Feb;
[PubMed PMID: 30445121]
[19]
Iñigo Martínez J,Gil Montalbán E,Jiménez Bueno S,Martín Martínez F,Nieto Juliá A,Sánchez Díaz J,García Marín N,Córdoba Deorador E,Nunziata Forte A,Alonso García M,Humanes Navarro AM,Montero Morales L,Domínguez Rodríguez MJ,Carbajo Ariza M,Díaz García LM,Mata Pariente N,Rumayor Zarzuelo M,Velasco Rodríguez MJ,Aragón Peña A,Rodríguez Baena E,Miguel Benito Á,Pérez Meixeira A,Ordobás Gavín M,Lopaz Pérez MÁ,Arce Arnáez A, Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2022 Jul;
[PubMed PMID: 35801519]
[20]
Selb R,Werber D,Falkenhorst G,Steffen G,Lachmann R,Ruscher C,McFarland S,Bartel A,Hemmers L,Koppe U,Stark K,Bremer V,Jansen K,Berlin MPX study group., A shift from travel-associated cases to autochthonous transmission with Berlin as epicentre of the monkeypox outbreak in Germany, May to June 2022. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2022 Jul;
[PubMed PMID: 35801518]
Level 3 (low-level) evidence
[21]
Antinori A,Mazzotta V,Vita S,Carletti F,Tacconi D,Lapini LE,D'Abramo A,Cicalini S,Lapa D,Pittalis S,Puro V,Rivano Capparuccia M,Giombini E,Gruber CEM,Garbuglia AR,Marani A,Vairo F,Girardi E,Vaia F,Nicastri E,INMI Monkeypox Group., Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2022 Jun;
[PubMed PMID: 35656836]
Level 2 (mid-level) evidence
[22]
Luna N,Ramírez AL,Muñoz M,Ballesteros N,Patiño LH,Castañeda SA,Bonilla-Aldana DK,Paniz-Mondolfi A,Ramírez JD, Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: Emergence of a novel viral lineage? Travel medicine and infectious disease. 2022 Sep-Oct
[PubMed PMID: 35840078]
[23]
Grant R,Nguyen LL,Breban R, Modelling human-to-human transmission of monkeypox. Bulletin of the World Health Organization. 2020 Sep 1;
[PubMed PMID: 33012864]
[24]
Hutson CL,Carroll DS,Gallardo-Romero N,Drew C,Zaki SR,Nagy T,Hughes C,Olson VA,Sanders J,Patel N,Smith SK,Keckler MS,Karem K,Damon IK, Comparison of Monkeypox Virus Clade Kinetics and Pathology within the Prairie Dog Animal Model Using a Serial Sacrifice Study Design. BioMed research international. 2015;
[PubMed PMID: 26380309]
Level 3 (low-level) evidence
[25]
McCollum AM,Damon IK, Human monkeypox. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 Jan;
[PubMed PMID: 24158414]
[26]
Weaver JR,Isaacs SN, Monkeypox virus and insights into its immunomodulatory proteins. Immunological reviews. 2008 Oct;
[PubMed PMID: 18837778]
[27]
Orviz E,Negredo A,Ayerdi O,Vázquez A,Muñoz-Gomez A,Monzón S,Clavo P,Zaballos A,Vera M,Sánchez P,Cabello N,Jiménez P,Pérez-García JA,Varona S,Del Romero J,Cuesta I,Delgado-Iribarren A,Torres M,Sagastagoitia I,Palacios G,Estrada V,Sánchez-Seco MP,Grupo Viruela del Simio Madrid CNM/ISCIII/HCSC/Sandoval., Monkeypox outbreak in Madrid (Spain): Clinical and virological aspects. The Journal of infection. 2022 Oct
[PubMed PMID: 35830908]
[28]
Martín-Delgado MC,Martín Sánchez FJ,Martínez-Sellés M,Molero García JM,Moreno Guillén S,Rodríguez-Artalejo FJ,Ruiz-Galiana J,Cantón R,De Lucas Ramos P,García-Botella A,García-Lledó A,Hernández-Sampelayo T,Gómez-Pavón J,González Del Castillo J,Muñoz P,Valerio M,Catalán P,Burillo A,Cobo A,Alcamí A,Bouza E, Monkeypox in humans: a new outbreak. Revista espanola de quimioterapia : publicacion oficial de la Sociedad Espanola de Quimioterapia. 2022 Jul 6;
[PubMed PMID: 35785957]
[29]
Hussey HS,Abdullahi LH,Collins JE,Muloiwa R,Hussey GD,Kagina BM, Varicella zoster virus-associated morbidity and mortality in Africa: a systematic review protocol. BMJ open. 2016 Apr 20;
[PubMed PMID: 27098823]
Level 1 (high-level) evidence
[30]
Osadebe L,Hughes CM,Shongo Lushima R,Kabamba J,Nguete B,Malekani J,Pukuta E,Karhemere S,Muyembe Tamfum JJ,Wemakoy Okitolonda E,Reynolds MG,McCollum AM, Enhancing case definitions for surveillance of human monkeypox in the Democratic Republic of Congo. PLoS neglected tropical diseases. 2017 Sep;
[PubMed PMID: 28892474]
Level 3 (low-level) evidence
[31]
Aden TA,Blevins P,York SW,Rager S,Balachandran D,Hutson CL,Lowe D,Mangal CN,Wolford T,Matheny A,Davidson W,Wilkins K,Cook R,Roulo RM,White MK,Berman L,Murray J,Laurance J,Francis D,Green NM,Berumen RA 3rd,Gonzalez A,Evans S,Hudziec M,Noel D,Adjei M,Hovan G,Lee P,Tate L,Gose RB,Voermans R,Crew J,Adam PR,Haydel D,Lukula S,Matluk N,Shah S,Featherston J,Ware D,Pettit D,McCutchen E,Acheampong E,Buttery E,Gorzalski A,Perry M,Fowler R,Lee RB,Nickla R,Huard R,Moore A,Jones K,Johnson R,Swaney E,Jaramillo J,Reinoso Webb C,Guin B,Yost J,Atkinson A,Griffin-Thomas L,Chenette J,Gant J,Sterkel A,Ghuman HK,Lute J,Smole SC,Arora V,Demontigny CK,Bielby M,Geeter E,Newman KAM,Glazier M,Lutkemeier W,Nelson M,Martinez R,Chaitram J,Honein MA,Villanueva JM, Rapid Diagnostic Testing for Response to the Monkeypox Outbreak - Laboratory Response Network, United States, May 17-June 30, 2022. MMWR. Morbidity and mortality weekly report. 2022 Jul 15;
[PubMed PMID: 35834423]
[33]
Wittek R, Vaccinia immune globulin: current policies, preparedness, and product safety and efficacy. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2006 May
[PubMed PMID: 16564720]
[34]
Reynolds MG,McCollum AM,Nguete B,Shongo Lushima R,Petersen BW, Improving the Care and Treatment of Monkeypox Patients in Low-Resource Settings: Applying Evidence from Contemporary Biomedical and Smallpox Biodefense Research. Viruses. 2017 Dec 12;
[PubMed PMID: 29231870]
[35]
Vivancos R,Anderson C,Blomquist P,Balasegaram S,Bell A,Bishop L,Brown CS,Chow Y,Edeghere O,Florence I,Logan S,Manley P,Crowe W,McAuley A,Shankar AG,Mora-Peris B,Paranthaman K,Prochazka M,Ryan C,Simons D,Vipond R,Byers C,Watkins NA,UKHSA Monkeypox Incident Management team.,Welfare W,Whittaker E,Dewsnap C,Wilson A,Young Y,Chand M,Riley S,Hopkins S,Monkeypox Incident Management Team., Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2022 Jun
[PubMed PMID: 35656834]