Continuing Education Activity
A pediatric cataract is an important cause of treatable childhood blindness. The condition must be recognized and managed early to optimize outcomes. Leukocoria, strabismus, and nystagmus are common presenting signs. Pediatric cataracts may be congenital, arising from genetic or pregnancy-related conditions, such as maternal infections, or acquired during the postnatal period.
Various leukocoria differentials must be ruled out before managing pediatric cataracts. A complete clinical evaluation is essential to identify the disease. Diagnostic tests such as the slit-lamp and ultrasonography help determine the cause. Treatment depends on the etiology.
This activity for healthcare professionals is designed to enhance learners' proficiency in evaluating and managing pediatric cataracts. Participants gain a deeper understanding of this condition's etiology, pathophysiology, evaluation, management, and postoperative rehabilitation. Greater competence enables learners to collaborate with interprofessional teams caring for children with this condition.
Objectives:
Identify the signs and symptoms of pediatric cataracts.
Develop a clinically appropriate diagnostic plan for a child with a suspected cataract.
Determine the most suitable management options for a child diagnosed with cataracts.
Implement effective collaboration and communication among interprofessional team members to improve outcomes and treatment efficacy for patients with pediatric cataracts.
Introduction
Pediatric cataracts are one of the leading causes of treatable childhood blindness. Untreated cataracts can significantly impact the affected child, family, and society at large socially, economically, and emotionally. Pediatric cataracts remain a challenge in ophthalmological practice because of the need to identify, diagnose, and manage the condition as early as possible to prevent amblyopia.[1]
Routine screening and awareness among parents about leukocoria and strabismus lead to early diagnosis and management. A good preoperative evaluation, intraocular lens (IOL) power calculation, meticulous surgery, and equally efficient postoperative care and visual rehabilitation are important for a favorable outcome. An interdepartmental effort involving pediatrics, anesthesia, ophthalmology, and optometry helps properly and effectively manage pediatric cataracts.[2][3][4]
Pediatric cataracts are a major cause of preventable blindness worldwide, especially prevalent in developing countries where late diagnosis often results in symptoms such as nystagmus, poor fixation, and complete cataracts. Early intervention can significantly improve affected children's personal and social lives, enhancing their visual rehabilitation and positively impacting their family's socioeconomic status. Pediatric cataract contributes to 5% to 20% of childhood blindness and severe visual impairment globally, with an incidence rate between 1.8 and 3.6 per 10,000 children annually. The global prevalence ranges from 1 to 15 per 10,000 children.[5]
Holmes et al found an estimated prevalence of 3 to 4 visually significant cataracts per 10,000 live births in the United States.[6] Similarly, a study by Rahi et al in the United Kingdom reported a prevalence of 3.18 per 10,000 live births. Nile et al reported an incidence of approximately 5 per 10,000 births in China. Despite geographic variations, significant laterality or gender differences are not commonly reported.[7]
Hereditary congenital cataracts have a prevalence between 8.3% and 25%, with about 75% of cases following an autosomal dominant inheritance pattern. Mutations in crystallin protein, essential for the lens's transparency and refractive power, are linked to various cataract types, such as nuclear, lamellar, zonular, and posterior polar cataracts. Nonsyndromic inherited cataracts often involve crystallin and connexin gene mutations, with PITX3 mutations linked to posterior polar cataracts and PAX6 to anterior polar cataracts.[8]
Syndromic cataracts are associated with genetic mutations, including galactosidase-α i Fabry disease, galactose-1-phosphate uridyltransferase (GALT) in galactosemia, OCRL (oculocerebrorenal syndrome of Lowe) in Lowe syndrome, and NHS in Nance–Horan cataract–dental syndrome (see Image. Congenital Cataracts and Abnormal Galactose Metabolism). Maternal and congenital infections like Toxoplasma gondi, Rubella, Cytomegalovirus, Herpes, and Syphilis (TORCHES) are also significant contributors to pediatric cataracts, with B Mahalakshmi et al reporting a high incidence of TORCH infections in the Indian subcontinent, finding 20% of cases seropositive. Trauma is another major cause, accounting for 12-46% of pediatric cataracts.[9]
Concerns exist regarding the higher incidence of complications, such as glaucoma, uveitis, dense posterior capsule opacification (PCO), and increased secondary interventions following primary intraocular lens (IOL) implantation in children younger than 2 years.[10] However, primary IOL implantation in these children has been shown to be safe, with excellent long-term outcomes compared to aphakia and secondary IOL implantation after age 2. The myopic shift is well-controlled, resulting visual acuity is generally good, and complications such as glaucoma, uveitis, membrane formation, synechia, and secondary interventions are less frequent. Special care is necessary for children younger than 6 months due to the high risk of adverse events in smaller eyes.[11]
The process of emmetropisation in children’s eyes is typically completed by age 12, with axial length increasing from an average of 16.5 mm at birth to 23 mm by age 13. This process can be divided into the rapid (0.46mm/month from birth to 6 months), infantile (0.15mm/month from 6 to 18 months), and juvenile phase (from 18 months to 12 years). The eye's axial length increases from 16.5 mm at birth to 23 mm by age 13 on average.
Corneal curvature and mean keratometry readings also change from 51.2D at birth to 43.5D in adults. Consequently, the power of the IOL implanted in children must be adjusted to account for the myopic shift and corneal curvature changes that occur with age. The adjustment necessitates customized IOL power calculation formulas for growing pediatric eyes. Sharp-edged IOLs are now widely accepted due to their association with lower rates of visual opacification, requiring fewer Nd YAG capsulotomies compared to round-edged IOLs (1/371 versus 4/371).
Managing pediatric cataracts is crucial, as timely intervention is essential for optimal visual recovery. Most children with congenital or developmental cataracts require surgical intervention. The impact of cataracts on visual acuity can be assessed using the red reflex observed during distant direct ophthalmoscopy (see Image. Red Reflex). Bilateral visually significant cataracts should be removed between 6 to 8 weeks of age, while unilateral visually significant cataracts should be addressed between 4 to 6 weeks.[12]
Etiology
The causes of pediatric cataracts are diverse and can range from being idiopathic to being associated with systemic disorders. Pediatric cataracts can be unilateral or bilateral, with most unilateral and some bilateral cases being idiopathic.
Causes of Congenital Cataracts
- Idiopathic
- Intrauterine infection (TORCH infections) [13]
- Drug-induced
- Metabolic disorders[14]
- Galactosemia
- Galactokinase deficiency
- Hypocalcemia
- Hypoglycemia
- Trauma
- Accidental
- Non-accidental
- Radiation
- Laser photocoagulation
- Other associated ocular diseases
- Microphthalmia, Microcornea
- Aniridia
- Persistent hyperplastic primary vitreous (PHPV)
- Peter anomaly
- Corneal guttae
- Coloboma
- Inherited without systemic abnormalities [15]
- Autosomal dominant (most common)
- Autosomal recessive (mostly seen in families with a history of consanguinity)
- X-linked
- Associated with systemic abnormalities
- Chromosomal abnormalities
- Trisomy 21
- Turner syndrome
- Trisomy 13
- Trisomy 18
- Cri-du-chat syndrome
- Cerebrooculofacialskeletal syndrome (COFS)
- Mitochondrial abnormalities
- Renal disease
- Skeletal disease
- Smith-Lemli-Opitz
- Conradi syndrome
- Weill-Marchesani syndrome
- Syndactyly, polydactyly, or digital abnormalities
- Bardet-Biedl syndrome
- Rubenstein-Taybi syndrome
- Central nervous system abnormalities
- Zellweger syndrome
- Meckel-Gruber syndrome
- Cardiac disease
- Hypertrophic cardiomyopathy
- Dermatological
- Cockayne syndrome
- Rothmund-Thomson
- Atopic dermatitis
- Incontinentia pigmenti
- Progeria
- Ichthyosis
- Ectodermal dysplasia
- Dental Anomalies
- Nance-Horan syndrome
- Lenz syndrome
Causes of Bilateral Congenital Cataracts
- Idiopathic Causes (60%)
- Hereditary Causes (30%)
- Autosomal Dominant (75%, most common)
- Autosomal Recessive
- X-linked
- Intrauterine infection (TORCH infections- toxoplasma, CMV retinitis, rubella, HSV)
- Multisystem genetic disorder
- Inborn errors of metabolism
- Endocrinopathies
- Trauma
- Uveitic cataract
- Ocular Abnormalities
- Genetic Conditions
- Down Syndrome
- Turner Syndrome
- Myotonic Dystrophy
- Metabolic Disorders
- Diabetes Mellitus (vacuolar cataracts)
- Hypoglycemia
- Hypocalcemia
- Galactosemia (oil droplet cataracts)
- Fabry Disease (spoke-like cataracts)
- Zellweger Syndrome
- Hypoparathyroidism (multicolored flecks)
- Lowe Syndrome (thin disciform cataracts)
- Drug-Induced Cataracts
- Steroids
- Miotics
- Chlorpromazine
- Amiodarone
Pediatric cataracts exhibit a wide range of etiologies and manifestations. A thorough evaluation is thus necessary to identify underlying causes, which can significantly impact management and treatment strategies. Recognizing whether the cataracts are unilateral or bilateral and understanding the potential systemic associations are crucial for providing comprehensive care and improving outcomes for affected children.
Epidemiology
Various epidemiological studies have been performed to evaluate the causes of childhood blindness. Untreated pediatric cataracts account for about 7.4% to 15.3% of childhood blindness. The incidence and prevalence of pediatric cataracts differ from region to region, being lower in high and higher in low socioeconomic countries. The incidence ranges between 1.8 to 3.6/10000 per year, while prevalence varies between 0.63 to 13.6 per 10000 in low-income economies like Bangladesh, Pakistan, and India to 0.42 to 2.05/10000 in high-income economies like the US and UK.[16] Pediatric cataracts exhibit no prevalence differences based on sex or laterality.[17]
Pediatric cataracts are a significant cause of treatable childhood blindness globally, with higher incidence rates observed in developing countries. The condition often leads to severe visual impairment if not promptly addressed, impacting both the child's quality of life and the socioeconomic status of their family and community. Pediatric cataracts account for 5% to 20% of childhood blindness and severe visual impairment worldwide. The incidence of pediatric cataracts ranges from 1.8 to 3.6 per 10,000 children annually. Global prevalence is estimated to be between 1 to 15 per 10,000 children. Studies from different countries report varied prevalence rates: In the United States, the prevalence is estimated to be 3-4 visually significant cataracts per 10,000 live births.[18]
The prevalence of pediatric cataracts in the United Kingdom is approximately 3.18 per 10,000 live births. The incidence in China is around 5 per 10,000 births. Geographic variations in the prevalence of pediatric cataracts exist, but significant differences in laterality (whether the cataract affects 1 or both eyes) or gender are not commonly reported.
Hereditary cataracts constitute about 30% of all pediatric cataract cases. Approximately 75% of these cases follow an autosomal dominant inheritance pattern, with others being autosomal recessive or X-linked. Specific genetic conditions associated with pediatric cataracts include Down syndrome, Turner syndrome, and myotonic dystrophy. Various metabolic disorders contribute to the development of pediatric cataracts, such as diabetes mellitus, hypoglycemia, hypocalcemia, galactosemia, Fabry disease, Zellweger syndrome, hypoparathyroidism, and Lowe syndrome.[19]
Certain medications, including steroids, miotics, chlorpromazine, and amiodarone, can induce cataracts in children. Trauma is another significant cause, accounting for 12% to 46% of all pediatric cataract cases. Maternal and congenital infections, including those from the TORCHES group, are notable contributors to pediatric cataracts. A study in the Indian subcontinent reported a high incidence of TORCH infections, with 20% of cases being seropositive. Pediatric cataracts are a critical public health issue requiring timely diagnosis and intervention to prevent long-term visual impairment. Understanding the epidemiology, including genetic, metabolic, drug-induced, and infectious causes, is essential for effective management and treatment strategies.[20]
Pathophysiology
Pediatric cataracts have diverse causes, including heritable genetic defects, fetal or childhood developmental insults, and associated systemic syndromes. The autosomal dominant inheritance type is commonly seen in hereditary cataracts. A genetic screening study in Australia identified as many as 51 genes and loci.[21] Mutations in genes coding for transcription proteins like PAX6, FoxE3, C-MAF, PITX3, MIP, and CRYAA are frequent. Crystallin and connexin mutations are also seen in most cases.[22][23]
Pediatric cataracts cloud the eye's natural lens, impairing vision. The multifaceted pathophysiology involves genetic, metabolic, infectious, and environmental factors that disrupt the lens's normal transparency and function.
Genetic factors play a crucial role in pediatric cataracts, with a significant proportion being hereditary and often following an autosomal dominant pattern. Mutations in genes responsible for the structure and function of lens proteins, such as crystallins, connexins, and transcription factors, lead to lens opacification. Crystallins maintain lens transparency and refractive properties, and mutations in these proteins can cause various cataracts, including nuclear, lamellar, zonular, and posterior polar cataracts. Connexins are essential gap junction proteins for cell communication within the lens. Mutated connexins disrupt lens cell homeostasis and transparency. Transcription factors, such as PITX3 and PAX6, are also critical. Mutations in these genes are linked to posterior polar and anterior polar cataracts, respectively, affecting lens development and maintenance.
Metabolic disorders significantly contribute to pediatric cataracts. Diabetes mellitus causes high blood glucose levels, leading to osmotic imbalances in the lens that result in swelling and vacuole formation, contributing to cataract formation. In galactosemia, the accumulation of galactitol due to deficient galactose metabolism causes osmotic stress and the formation of oil droplet cataracts. Hypocalcemia, characterized by low calcium levels, can disrupt lens cell function and protein stability, leading to cataractogenesis. Fabry disease, a lysosomal storage disorder, results in the accumulation of glycosphingolipids, causing spoke-like cataracts.[24]
Maternal and congenital infections, particularly TORCH infections, can lead to congenital cataracts in newborns. These infections cause inflammation and damage the developing lens during pregnancy, producingf lens opacification.[25]
Drug-induced cataracts can result from prolonged use of certain medications. Corticosteroids, for example, can alter lens metabolism and protein structure, leading to posterior subcapsular cataracts. Other medications, such as miotics, chlorpromazine, and amiodarone, can cause lens opacities through mechanisms like oxidative stress and interference with lens cell function. Trauma to the eye, whether accidental or intentional, can also cause cataracts. Mechanical injury disrupts the lens capsule, allowing fluid entry and protein denaturation, resulting in cataract formation.[26]
Environmental and nutritional factors play significant roles in the development of pediatric cataracts. Prolonged exposure to ultraviolet radiation and other environmental stressors can heighten oxidative stress in the lens. This increased oxidative stress damages proteins and lipids within the lens, ultimately forming cataracts. Additionally, deficiencies in essential nutrients, particularly antioxidants like vitamins C and E, can compromise the overall health of the lens. Such deficiencies weaken the lens's ability to combat oxidative damage and contribute to the progression of cataracts.[27]
The mechanisms underlying lens opacification are multifaceted and encompass various biological processes. Protein aggregation, resulting from mutations or metabolic imbalances, causes crystallin proteins to unfold and aggregate, leading to light scattering and lens opacity. Osmotic imbalances, seen in conditions like diabetes and galactosemia, prompt the accumulation of osmotic agents within the lens, triggering cell swelling, membrane rupture, and protein leakage. Calcium homeostasis disruptions further contribute to lens opacity by affecting cell signaling and protein stability.[28]
Inflammatory responses elicited by infections or trauma can damage lens cells and proteins, further exacerbating cataract formation. Given the complexity of pediatric cataracts' pathophysiology, which involves genetic mutations, metabolic disturbances, infections, drug effects, trauma, and environmental influences, comprehending these mechanisms is essential for devising effective preventive and therapeutic interventions to preserve vision in affected children.[29]
Histopathology
The histopathology of pediatric cataracts involves the microscopic examination of lens tissue to understand the structural changes that lead to opacification. Various types of cataracts exhibit distinct histopathological features, which provide insights into their underlying mechanisms and potential treatment approaches.
Pediatric cataracts often show disorganized and fragmented lens fibers. Normal lens fibers are tightly packed and transparent, but in cataractous lenses, they can be swollen, misaligned, and vacuolated. Aggregates of denatured or misfolded proteins, such as crystallins, can be observed within lens fibers. These protein aggregates scatter light, leading to lens opacity. Fluid-filled vacuoles are common in various pediatric cataracts, including those associated with metabolic disorders. These vacuoles result from osmotic imbalances and lens cell damage.[30]
Genetic cataracts manifest in various forms, each with distinct characteristics and histological features. Nuclear cataracts, for instance, are marked by central lens opacity, featuring dense protein aggregation and heightened lens fiber compaction within the nucleus. On the other hand, lamellar cataracts appear as disk-shaped opacities affecting specific layers of the lens. Histologically, these layers exhibit disrupted and swollen lens fibers with protein aggregates. Posterior polar cataracts localize at the posterior aspect of the lens, involving alterations in the posterior capsule and underlying lens fibers. These alterations encompass fibrosis and abnormal cell proliferation, contributing to the opacity observed in this type of cataract.
Metabolic cataracts encompass a spectrum of conditions linked to metabolic disturbances, each exhibiting distinctive pathological features. Cataracts associated with galactosemia, for instance, are characterized by oil droplet formations within the lens, showcasing vacuoles and osmotic swelling in lens fibers alongside deposits of galactitol. In diabetic cataracts, hallmark features include vacuolar changes, lens fiber swelling, and sorbitol accumulation. The osmotic effect induced by sorbitol prompts water influx into the lens, contributing to opacity. Fabry disease-associated cataracts often display spoke-like opacities, accompanied by lipid accumulation and abnormal glycosphingolipid deposits within lens fibers. These distinct manifestations underscore the intricate relationship between metabolic abnormalities and lens pathology in metabolic cataracts.[31]
Congenital infections, particularly those associated with TORCH pathogens, leave characteristic histopathological imprints on the lens. Examination often reveals signs of inflammation, fibrosis, and cellular infiltration within the lens tissue. Thickening of the lens capsule may also be observed, along with evidence of viral or parasitic inclusion bodies, further underscoring the impact of congenital infections on lens health and development.
Traumatic cataracts arise from physical injury to the eye, eliciting various pathological changes within the lens. Capsule rupture, subsequent fibrosis, and abnormal cell proliferation commonly occur posttrauma. This disruption in the lens capsule's integrity often results in disorganized lens fibers and subsequent opacity. Additionally, calcium deposition within the lens, observed in some cases of traumatic cataracts, further exacerbates opacity and contributes to lens rigidity. These pathological alterations highlight the diverse effects of trauma on lens structure and function in traumatic cataracts.[32]
Certain medications can cause cataracts. Steroid-induced cataracts typically show posterior subcapsular opacities with swollen and vacuolated lens epithelial cells on histopathology. These changes are primarily due to altered cellular metabolism and protein aggregation. Other drugs that can cause cataracts include Miotics, chlorpromazine, and amiodarone, which cause similar histopathological changes, including lens fiber swelling, vacuole formation, and protein aggregation.[33]
Key histopathological techniques include light and electron microscopy and immunohistochemistry. Light microscopy examines stained lens tissue sections, revealing structural changes in lens fibers, protein aggregates, and cellular abnormalities. Electron microscopy provides detailed images of subcellular structures, allowing for the observation of crystallin protein aggregates, cell membrane disruptions, and intracellular vacuoles. Immunohistochemistry detects specific proteins and cellular markers, helping to identify genetic mutations and metabolic disturbances contributing to cataract formation.[34]
The histopathology of pediatric cataracts reveals a variety of structural and cellular abnormalities that contribute to lens opacification. Understanding these changes at the microscopic level is essential for diagnosing the underlying causes of cataracts and developing targeted treatments to restore vision in affected children.
History and Physical
Pediatric cataract evaluation requires a meticulous approach, integrating a comprehensive history, thorough ocular examination, and systemic assessment. This multifaceted approach is crucial to rule out potential underlying causes and associated ocular or systemic conditions.
History taking should elicit key details, such as the age of onset, duration of symptoms, antenatal and perinatal history (including maternal infections or exposures and any birth complications), developmental history with a focus on milestones achieved, any behavioral changes suggesting visual dysfunction (eg, inability to catch objects, frequent falls, and light sensitivity), history suggestive of systemic abnormalities, trauma, previous treatment or surgery, and family history of congenital or developmental cataracts or consanguinity.[35]
A meticulous physical examination is crucial for evaluating pediatric cataracts. The pediatrician can rule out any systemic or genetic associations with cataracts with careful physical inspection. For instance, head circumference measurement is particularly important for congenital cataracts, as some syndromes, like trisomy 21 and Hallermann-Streiff-Francois, Lowe's oculocerebrorenal, Cri-du-chat (5p deletion), Nance-Horan, and Edward syndromes, can manifest with both cataracts and abnormal head size.[36]
Reviewing family photos can be helpful if the exact onset of cataracts is unclear. Early cataracts developing during critical ocular and visual development stages may have a poorer prognosis.[37] Finally, examining the eyes of family members, especially parents and siblings, can reveal undiagnosed lenticular changes suggesting inherited causes of pediatric cataracts. Autosomal dominant inheritance is most common, but X-linked recessive patterns can also occur in conditions like Lowe syndrome and Nance-Horan syndrome.[38][39]
A comprehensive ocular examination is crucial for pediatric cataract assessment. Visual acuity testing employs various age-appropriate methods. In preverbal children, tools that may be used include the Bruckner test, cover test, resistance to occlusion of the better eye, monocular fixation, fixation preference, vertical prism test, forced-choice preferential looking, Teller acuity cards, optokinetic nystagmus, and pattern visual evoked potential.[40][41]
The Bruckner test is performed with a direct ophthalmoscope, allowing simultaneous visualization of the red reflex in both eyes. This technique can reveal potential anisometropia or unequal refractive power between the eyes. In cases of strabismus, the deviated eye often exhibits a lighter red reflex than the fixating eye. Similarly, with anisometropia, one eye's red reflex may appear brighter than the other.
Verbal children undergo a multifaceted visual acuity assessment. Detection acuity testing, employing tools like the Catford drum test and Standardized Tests for Young Children and Retardates (STYCAR) graded ball test, evaluates the ability to perceive light or motion.[42]
Recognition acuity testing assesses the ability to recognize specific stimuli, encompassing direction identification using Landolt C or Snellen E tests, letter identification with Snellen charts or high-contrast optotype visual acuity (HOTV) tests, and picture identification with tools like Allen picture cards or Domino charts. Behavioral pattern assessment, through techniques like the Cardiff acuity test and optokinetic drum test, evaluates visual function indirectly by observing the child's response to visual stimuli.[43]
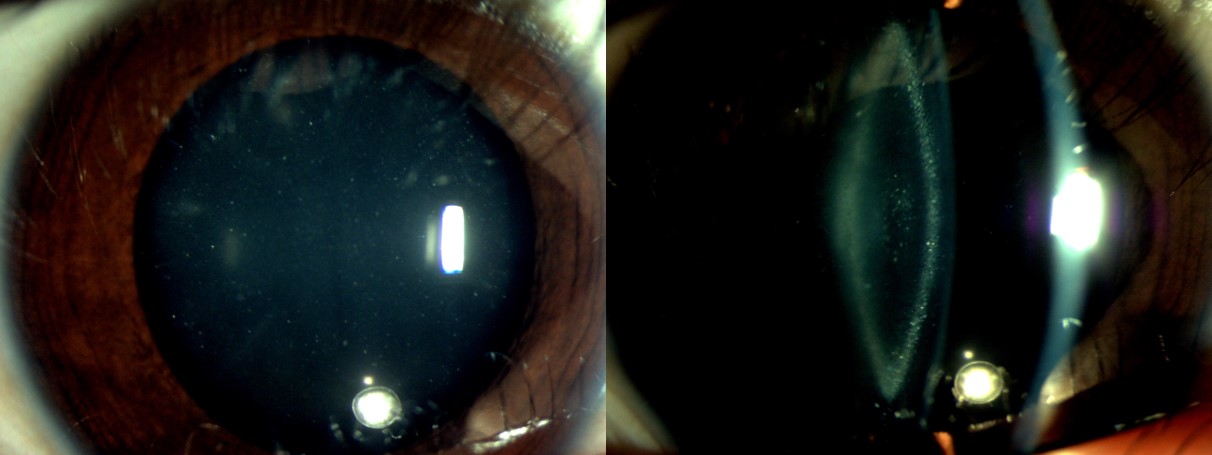
The slit-lamp examination assesses for any associated anterior segment findings, such as microcornea, anterior segment dysgenesis, iris coloboma, cataract morphology, any microspherophakia, ectopia lentis or lens subluxation, and preexisting posterior capsular defect (see Image. Slit-Lamp Examination of Pediatric Zonular Cataract). The morphological classification of pediatric cataracts is as follows:[44]
- Anterior cataracts: anterior lenticonus (associated with Alport syndrome), anterior polar, anterior pyramidal, and anterior subcapsular
- Cataract involving whole lens: total and membranous cataracts
- Posterior cataracts: Mittendrofs dot, posterior lenticonus, and posterior subcapsular cataract (see Image. Pediatric Cataract, Posterior Lenticonus)
- Central cataract: Lamellar (zonular), pulverulent, oil drop, coronary, and blue dot cataract
- Sutural cataract
- Wedge-shaped cataract
Ocular motility evaluation investigates strabismus and nystagmus, which should be specifically assessed in children. These manifestations are sometimes the presenting signs of pediatric cataracts and potential visual impairment. Strabismus is usually seen in children with unilateral cataracts and develops when an irreparable visual loss has already occurred. Congenital sensory nystagmus is usually associated with pediatric cataracts. The presence of either strabismus or nystagmus indicates that cataracts are visually significant.[45][46][47]
Intraocular pressure measurement rules out glaucoma associated with congenital rubella syndrome with the help of a tonometer.[48] Pupillary reaction testing can give a rough idea about the health of the optic nerve head.
Direct and indirect ophthalmoscopy evaluates for associated vitreous or posterior segment abnormality, such as vitreous hemorrhage, primary vitreous remnants, fundal coloboma, and optic or macular hypoplasia.
Visually significant cataracts may be centrally or posteriorly located, with sizes greater than 3 mm. These cataracts usually produce a visual acuity of or below 20/60, decreased contrast sensitivity, increased glare, loss of stereo acuity, and associated strabismus or nystagmus.[49][50]
Examination of The Red Reflex
Medical professionals caring for children, especially newborns and neonates, must be familiar with performing the red reflex test. The American Academy of Pediatrics (AAP) mandates that red reflex testing should be performed on all neonatal patients and at all routine well-child examinations and healthcare appointments.[51][52][53][54]
The red reflex is commonly seen in photographs taken with a flash, essentially a reflective phenomenon. The cornea reflects light that enters the eye through the pupil, creating a red glow. The presence of a red reflex confirms transparency in the eye's optical structures, including the tear film, the cornea, the aqueous humor, the lens, the vitreous gel, and the retina.[55] The red reflex is performed with emphasis on the reflex's presence or absence, color, brightness, and symmetry between the eyes.[56]
Requirements to perform a red reflex test include the following:
- Dimming or turning off the room lights
- Having the child on the parent's lap so that the height of the child's eyes is aligned with those of the examiner
- Setting the direct ophthalmoscope diopter power to that of the examiner's eye reading or to "0"
- Holding the ophthalmoscope close to the examiner's eye and 12 to 18 inches away from those of the child after being turned on
- Encouraging the child to look at the light, preferably with the aid of an assistant and toys as needed
- Directing the light at one eye and then the other to detect and compare the light reflexes
- Examining the symmetry by signing the light over both eyes (Bruckner test) [57]
The red reflex examination's simplicity, speed, and noninvasiveness make it an essential tool for the early detection of pediatric cataracts.
Evaluation
Laboratory investigations encompass blood and urine examinations and other tests. Blood and routine investigations should include a complete hemogram, blood glucose, and urine routine microscopy. Other tests are obtained on a case-to-case basis, depending upon the history and examination. These investigations include the following:
- Serum calcium for hyper or hypoparathyroidism [58]
- Venereal disease research laboratory testing for syphilis [59]
- TORCH infection antibody titers [60]
- Red cell galactokinase or uridyl transferase for galactosemia [61]
- Urine protein for Alport syndrome [62]
- Urine amino acid for Lowe syndrome
- Urine sodium nitroprusside or plasma homocysteine for homocystinuria [63]
- Urine or serum copper for Wilson disease [64]
- Karyotyping for a genetic defect
Ocular investigations for pediatric cataracts involve several key diagnostic tools. Ultrasound B scan is essential for excluding other posterior segment pathologies that can mimic congenital cataracts, such as retinoblastoma, persistent hyperplastic primary vitreous, Coats disease, retinopathy of prematurity with retrolental fibroplasia, organized vitreous hemorrhage, congenital falciform fold, ocular toxocariasis, and retinal hamartomas.[65][66] Additionally, ultrasound biometry provides crucial optical parameters like axial length, anterior chamber depth, and lens thickness, which are vital for intraocular lens (IOL) power calculation.[67][68]
Keratometry, typically performed using handheld keratometers, requires the child's cooperation for accurate readings. Standard K values of 43.00 D can be used if the child is uncooperative.[69] Optical coherence tomography is employed to assess retinal structure and identify associated pathologies. For systemic imaging, magnetic resonance imaging (MRI) or computed tomography (CT) scans are recommended when persistent fetal vasculature or retinoblastoma is suspected, as these modalities provide detailed imaging of the brain and orbits.
Additional evaluation considerations for pediatric cataracts include consultations with various specialists. For example, a pediatrician is essential to comprehensively evaluate the child's systemic health and coordinate overall care. A geneticist should be consulted for cases with suspected hereditary conditions to provide detailed genetic analysis and counseling. An endocrinologist should be involved when metabolic disorders affecting eye health are suspected, ensuring targeted management of these underlying conditions.[70]
Preoperative assessment for pediatric cataracts necessitates a comprehensive anesthetic evaluation to ensure the child is fit for anesthesia, particularly for infants and those with comorbid conditions. Managing pediatric cataracts requires an interprofessional approach, incorporating clinical, laboratory, and imaging assessments. Adhering to national and international guidelines standardizes diagnosis and management, improving outcomes for affected children. Early detection and thorough evaluation are crucial in preventing long-term visual impairment and enhancing the quality of life for these patients.
Treatment / Management
Managing pediatric cataracts requires team effort from ophthalmologists, pediatricians, anesthesiologists, counselors, parents, and other family members. Treatment options are either medical or surgical.
Medical Management
Medical management involves amblyopia therapy, such as patching the better-seeing eye to encourage the use of the amblyopic eye or using atropine drops to blur vision in the stronger eye as an alternative.[71] Refractive correction is achieved through glasses or contact lenses to address residual refractive errors postsurgery. Bifocals or multifocals may be needed for near-vision tasks.[72]
Low vision aids, including magnifiers and telescopic lenses, enhance visual function for children with significant visual impairment.[73] For mydriasis, 2.5% phenylephrine permits vision through the nonopacified area in cases of partial or non-amblyogenic cataracts. Optical iridectomy, although no longer used, served the same purpose as mydriatics by allowing vision through the nonopacified area.[74]
Surgery
Surgical indications include the presence of any visually significant opacity, cataract with visual acuity of or below 20/60, disc not visible with an indirect ophthalmoscope, central cataract at least 3 mm in size, posterior subcapsular cataract, nuclear cataract, bilateral cataract, and cataracts associated with strabismus and nystagmus.[75][76] Before surgery, the family members are counseled regarding the visual prognosis, the different available treatment options, and the need for regular follow-up, post-operative visual rehabilitation, and compliance with amblyopia therapy, if required.
Surgery should be performed immediately for visually significant cataracts, ideally within a few weeks after birth, to prevent amblyopia. Surgery is better planned after 4 years of age, as ocular development will be complete and postoperative complications will be less severe. Close follow-up and visual monitoring are required to prevent amblyopia and treat it as indicated. The consensus is to operate a unilateral cataract as early as 4 to 6 weeks after birth. Bilateral cataracts should be operated on by 6 to 8 weeks, and the second eye should be operated on within 2 weeks of the first surgery.
General anesthesia and constant monitoring of vital parameters are preferred for cataract surgery. A trained anesthesiologist team and interdepartmental support are required to deal with children.
The type of surgery and the surgical challenges have evolved significantly, with modern-day phacoaspiration and IOL implantation replacing discission. Choyce and Binkhorst first implanted a monocular intraocular lens in a child's eye in 1959. Pediatric cataract surgery differs from adult cataract surgery and presents various intraoperative challenges: low scleral rigidity complicates incision construction and wound closure, while the smaller eyeball size, shallow anterior chamber depth, and small pupil size reduce maneuverability. The elastic capsule, high positive intravitreal pressure, and risk of vitreous loss and expulsion of intraocular contents also add to the complexity.
The advantages of primary IOL implantation are immediate postoperative refractive correction, minimal or no optical aberration, full visual field, less chance of development and progression of amblyopia, strabismus, nystagmus, and minimal dependence on patients' compliance. IOL implantation in children younger than 2 years is still a controversial topic. Lack of long-term data to predict the success rate, associated other ocular abnormalities, cataracts, frequent occurrence of derivational amblyopia, and increased postoperative complications are the main reasons for limited uses of IOL implantation in children younger than 2 years. Infant Aphakia Treatment Study showed higher intraoperative and postoperative complications and the need for additional surgeries in children with IOL implantation before turning 1 year.[77]
A consensus regarding surgery performed and IOL implantation is as follows:[78]][79]
- Younger than age 18 months to 2 years: Lens aspiration without IOL implantation, with posterior curvilinear capsulorhexis (PCCC) and limited anterior vitrectomy (LAV). The child remains aphakic with postoperative aphakic correction, followed by secondary IOL implantation at a later age.
- 2 years to 5 years: Lens aspiration with IOL implantation with PCCC with LAV.
- Older than 8 years: Lens aspiration with IOL implantation with PCCC without LAV. Alternatively, phacoaspiration with IOL implantation may be performed like an adult cataract surgery.
Reducing the risk of visual axis opacification (VAO) requires that post-surgery PCCC is performed in children younger than 8 years and LAV is performed in children younger than 5 years.
Accurate IOL power calculation is crucial for achieving desired refractive outcomes after surgery in children. However, this process poses challenges, particularly in adjusting for the child's age and axial length of the eye. Biometry and keratometry measurements can vary depending on the instruments and methods used for measurement. Studies indicate that the SRK/T and Holladay 2 formulas exhibit the least predictive error among available IOL formulas. Ongoing research aims to develop newer IOL formulas to enhance the precision of IOL power calculation in children.
"Emmetropisation" refers to the adjustment of 3 main factors—axial length, corneal curvature, and lenticular power—toward adult values as a child grows. Axial length increases as the eyeball enlarges, leading to a myopic shift. Consequently, the initial target refractive outcome following IOL implantation is hypermetropia. Studies suggest that undercorrection of the required IOL power based on the child's age can improve postsurgery refractive outcomes. Dahan and Drusedau proposed undercorrection percentages: 20% for children younger than 2 years and 10% for children aged 2 to 8 years. The consensus is to aim for residual refraction of +6D (1 to 2 years), +5 (2 to 4 years), +4 (4 to 5 years), +3 (5 to 6 years), +2 (6 to 7 years), and Plano for children older than 14 years.[80][81][82][83]
National and International Guidelines
National and international guidelines provide comprehensive recommendations for detecting and managing pediatric cataracts. The AAP recommends red reflex examinations at every well-child visit starting from birth, with immediate referral to a pediatric ophthalmologist if an abnormal red reflex is detected. The American Academy of Ophthalmology (AAO) emphasizes early detection and treatment, especially for infants with visually significant cataracts, and advises genetic counseling and appropriate genetic testing for families with a history of pediatric cataracts. Postoperative amblyopia therapy and regular follow-up are recommended to monitor visual development and manage complications.[84][85][84] The World Health Organization advocates for the integrated management of childhood illnesses, including screening for congenital conditions like cataracts, and supports developing national programs to prevent and manage childhood blindness.[86][87]
Follow-Up Care
Follow-up care for pediatric cataracts involves several key aspects to ensure optimal outcomes. Regular ophthalmic evaluations are crucial, with frequent follow-ups to monitor for complications in the immediate postoperative period. Long-term follow-up tracks proper visual development and addresses any refractive changes.[88]
Developmental and educational support, including occupational therapy, is essential to aid in visual skills development and daily living activities. Special education services ensure appropriate accommodations for children with visual impairment.[89]
Parental education and support play a vital role, with detailed instructions provided on postoperative care, including administering eye drops and recognizing signs of complications. Emphasis is placed on the importance of adherence to amblyopia therapy and follow-up visits.[90]
Psychosocial support is offered, with access to counseling and support groups to help families cope with the emotional and psychological aspects of managing pediatric cataracts. This comprehensive and interprofessional approach, in line with national and international guidelines on early detection, timely surgical intervention, effective postoperative care, and ongoing visual rehabilitation, is essential for optimizing visual outcomes and enhancing affected children's overall quality of life.
Differential Diagnosis
Pediatric cataracts usually present with a whitish opacity in the eye, which the parents first notice. Various causes of the white reflex (leukocoria) must be ruled out before diagnosing a pediatric cataract alone. Causes of leukocoria include:[91]
- Corneal abnormalities (corneal opacity)
- Lens abnormalities (cataracts)
- Vitreous causes (eg, vitreous hemorrhage and persistent hyperplastic primary vitreous)
- Retinal diseases (eg, Coats disease, retinoblastoma, familial exudative vitreoretinopathy, retinal detachment, retinopathy of prematurity, and coloboma).
- Tumors such as medulloepithelioma and retinal astrocytoma [92]
Cataracts can occur in isolation or are associated with other causes of leukocoria. The differential diagnosis can be narrowed down based on history, family history, and a thorough ophthalmic examination.[93][94]
The differential diagnosis of pediatric cataracts also includes the following:
- Retinoblastoma
- Persistent Fetal Vasculature
- Coats disease
- Toxocariasis
- Retinal detachment [95]
- Optic nerve hypoplasia
- Congenital glaucoma [96]
- Retinal dysplasia
- Intraocular inflammation (uveitis) [97]
- Congenital rubella syndrome
- Norrie disease
- Leber's congenital amaurosis
- Familial exudative vitreoretinopathy (FEVR)
- Retinitis pigmentosa [98]
- Marfan syndrome [99]
- Lowe syndrome [100]
- Congenital syphilis
- Metabolic Disorders (eg, galactosemia and diabetes mellitus)
- Aniridia
- Coloboma [101]
- Stickler syndrome
- Bardet-Biedl syndrome
- Joubert syndrome
- Von Hippel-Lindau disease
- Wolfram syndrome
- Langerhans cell histiocytosis
- Oculocerebrorenal syndrome (Lowe syndrome)
- Alport syndrome
- Usher syndrome
- Wilms tumor, aniridia, genitourinary anomalies, and range of developmental delays (WAGR) syndrome
- Peter anomaly
- Axenfeld-Rieger syndrome
- Sturge-Weber syndrome
- Tuberculosis
- Cytomegalovirus (CMV) infection
- Toxoplasmosis
- Herpes simplex virus infection [102]
- Congenital varicella syndrome
- Retinal hemorrhages (eg, shaken baby syndrome)
- Albinism
- Blue cone monochromacy
- Best disease (Best vitelliform macular Dystrophy)
- Choroideremia
- Congenital stationary night blindness
- Bietti crystalline dystrophy
- Batten disease (neuronal ceroid lipofuscinosis)
- Usher syndrome
- Hyphema
- Sickle cell retinopathy
- Retinal vein occlusion
- Familial hypercholesterolemia (ocular manifestations)
- Fabry disease (ocular manifestations)
- Niemann-Pick disease
- Smith-Lemli-Opitz syndrome
- Congenital Zika syndrome
- Ehlers-Danlos syndrome (ocular manifestations)
- Ataxia-telangiectasia (ocular manifestations)
- Juvenile idiopathic arthritis (ocular manifestations)
- Neurofibromatosis type 1 (ocular manifestations)
- Hypocalcemia (ocular manifestations)
A thorough clinical investigation with the appropriate diagnostic modalities can guide management.
Staging
Pediatric cataracts can be staged based on their morphology, location, and impact on vision. Staging helps in planning appropriate treatment and management strategies. Below is an overview of the criteria commonly used for staging pediatric cataracts:
Morphological Classification
- Nuclear cataract: Located in the central nucleus of the lens. Often congenital and can significantly impair vision if dense.
- Cortical cataract: Affects the cortex or outer layer of the lens. May progress slowly. Vision impairment depends on the extent and location of opacities.
- Posterior subcapsular cataract: Positioned at the back of the lens just in front of the posterior capsule. This type can cause glare and difficulty seeing in bright light, significantly affecting near vision.
- Lamellar (zonular) cataract: Affects a specific layer or zone of the lens (see Image. Pediatric Cataract, Zonular Type). This type can cause substantial visual impairment if the opacity is dense and centrally located.
- Anterior polar cataract: Located at the front of the lens capsule. Typically small and may not significantly affect vision unless it enlarges.[103]
Severity Based on Density and Extent
- Mild cataract: Small opacities that do not significantly interfere with vision. Regular monitoring, possibly conservative management.
- Moderate cataract: More extensive opacities causing noticeable visual impairment. This type may require surgical intervention, particularly if vision is significantly affected.
- Severe cataract: These cataracts are dense opacities that severely impair vision or cause blindness. Prompt surgical intervention is typically needed to restore vision and prevent amblyopia.[104]
Impact on Vision
- Visually insignificant cataract: Does not significantly affect vision or causes only minor visual disturbances. Regular follow-up is essential to monitor for changes.
- Visually significant cataract: Causes noticeable visual impairment affecting daily activities and development. Requires timely surgical intervention to restore vision and ensure normal visual development.[105]
Location Relative to the Visual Axis
- Central cataract: Located in the central visual axis. More likely to interfere with vision and development, necessitating prompt treatment.
- Peripheral cataract: Located outside the central visual axis. This type may not significantly affect vision unless it progresses toward the center.
Laterality
- Unilateral cataract: The cataract affects only 1 eye. The affected eye is at a higher risk of amblyopia, requiring early intervention to prevent visual development issues.
- Bilateral cataracts: The cataracts affect both eyes. This condition can lead to bilateral visual impairment and requires coordinated surgical and rehabilitative efforts for both eyes.[106]
Staging pediatric cataracts involves assessing their morphology, severity, impact on vision, and location. This comprehensive approach helps devise appropriate treatment plans and interventions to ensure optimal visual outcomes and development for affected children. Regular monitoring and timely intervention are crucial to managing this condition effectively.
Prognosis
Numerous factors affect the final visual outcome in a child with a pediatric cataract. Visually significant cataract produces blurred images on the retina and thus affect the development of visual pathways and connections in the occipital cortex. Today, due to improved understanding and surgical techniques, early removal of visually significant cataracts is recommended to prevent sensory deprivation amblyopia. Unilateral cataracts are more amblyogenic than bilateral ones, emphasizing the need for early surgery within the first few weeks to months.
The time of diagnosis of a pediatric cataract also plays a crucial role in prognosticating the final visual outcome—the earlier the diagnosis, the earlier the treatment, the better the prognosis, and vice versa. Associated ocular diseases include microcornea, corneal opacities, glaucoma, intraocular inflammation, posterior segment abnormalities, and ocular movement disorders such as unsteady fixation, strabismus, nystagmus, or nystagmoid movements denote poorer prognosis postsurgery.[107]
The prognosis of pediatric cataracts largely depends on several factors, including the timing of diagnosis and intervention, the presence of any underlying systemic or genetic conditions, and the management of potential complications. Early detection and prompt treatment are crucial for achieving the best possible visual outcomes.
Early Diagnosis and Treatment
- Positive outcomes: Children diagnosed and treated early, preferably within the first few weeks to months of life, generally have a better prognosis. Early intervention helps to prevent amblyopia and allows for normal visual development.
- Delayed intervention: Delays in diagnosis and treatment can lead to permanent visual impairment, including amblyopia and other developmental issues, making early detection vital.[108]
Type and Severity of Cataract
- Isolated cataracts: Children with isolated cataracts, ie, not associated with other ocular or systemic abnormalities, often have a better prognosis if treated early.
- Associated conditions: Cataracts associated with other conditions, such as genetic syndromes or metabolic disorders, may have a more guarded prognosis due to the complexity of managing multiple health issues.
Surgical and Postoperative Management
- Surgical success: Modern surgical techniques, including the use of IOLs, have significantly improved the outcomes for children with pediatric cataracts. Successful cataract surgery, followed by appropriate visual rehabilitation, generally leads to good visual outcomes.
- Postoperative care: Effective postoperative care, including management of complications such as PCO and secondary glaucoma, is critical. Regular follow-ups and adherence to treatment protocols improve long-term visual prognosis.[109]
Visual Rehabilitation
- Corrective measures: Corrective lenses (glasses or contact lenses) and amblyopia therapy (such as patching) play a significant role in achieving good visual outcomes. Consistent use of these corrective measures is crucial for the child's visual development.
- Support and therapy: Ongoing support from occupational therapists and visual skills training can further enhance visual outcomes, especially in children who experience developmental delays due to vision impairment.
Long-Term Monitoring
Continued surveillance is essential to detect and manage any late-onset complications, such as glaucoma or retinal detachment, which can affect the prognosis. Regular eye examinations throughout childhood and adolescence ensure that any issues are addressed promptly.[110]
Individual Outcome Variability
Prognosis can vary widely among individuals. Factors such as the specific type of cataract, the child's overall health, the presence of other eye or health conditions, and how well the family follows postoperative care and rehabilitation plans all influence outcomes.
Overall, the prognosis for children with pediatric cataracts can be quite favorable with early detection, timely intervention, and comprehensive postoperative care. Advances in surgical techniques and visual rehabilitation have greatly improved outcomes, allowing many children to achieve good vision and lead normal lives. However, ongoing vigilance is necessary to manage potential complications and ensure long-term visual health.
Complications
As with any other intraocular surgery, pediatric cataract surgery has its own associated complications that may require additional treatment.
- VAO: This condition is a potential cause of amblyopia after cataract surgery.[111] VAO is inevitable if the posterior capsule is left intact. VAO results from the proliferation, migration, and metaplasia of lens epithelial cells from the equator to the posterior capsule. Even after adequate posterior capsulotomy, secondary membranes and media opacity can occur. An intact hyaloid face can serve as a scaffold for migrating lens epithelial cells and their subsequent proliferation and transformation. One of the crucial factors to influence is the patient's age during surgery. VAO also depends upon the type of cataract and associated ocular abnormalities (eg, PHPV, uveitis), aphakic or pseudophakic, type of IOL, size of the capsular opening, and completeness of cortical cleanup. Adequate posterior capsulotomy with or without vitrectomy is essential to prevent VAO. Implantation of IOL in the bag also reduces the chance of VAO. N:Yttrium-aluminum-garnet (Nd:YAG) laser capsulotomy can be done if the child is cooperative. A dense and thick VAO may also require additional surgery, like a membranectomy, to clear the visual axis.[112]
- Glaucoma: The incidence of glaucoma is 10% to 25%.[113] Open-angle glaucoma is more common, but pupillary block glaucoma can also occur. Medical management is usually sufficient, but surgical management is advised if glaucoma is uncontrolled. Surgical procedures like trabeculectomy, glaucoma drainage device implantation, and cyclodestructive procedures may be used.[114]
- Postoperative anterior uveitis: This condition is more common in children than adults due to increased tissue reactivity. No-touch surgery and in-bag fixation reduce it. Postoperative frequent application of topical steroids or posterior subtenon injection of triamcinolone acetonide may be needed. Sometimes, dense fibrinous exudates and postop miosis are seen. Mydriatics, such as atropine or even subconjunctival injected mydricaine, can be used in such cases to break synechiae formation and achieve mydriasis.
- IOL-related complications: IOL deposits, synechiae, pupillary capture, IOL opacification, and decentration can be seen in the postoperative period.
- IOL-related Issues: Complications related to IOL implantation can include incorrect IOL power, IOL dislocation, and inflammation. Accurate calculation of IOL power and proper surgical technique are essential. In case of dislocation or other issues, surgical intervention may be required to reposition or replace the IOL.
- Amblyopia: Amblyopia is a condition where vision in one eye does not develop properly, often due to unequal visual input. This condition is common in children with cataracts, particularly if the cataract is unilateral or dense. Early detection and treatment are essential. Interventions include patching the stronger eye and using atropine drops or corrective lenses to encourage the use of the weaker eye.[115]
- PCO: This condition is the most common postoperative complication. The lens capsule becomes cloudy, leading to decreased vision. Neodymium (Nd) laser capsulotomy is typically performed to create a clear visual axis and restore vision.[116]
- Strabismus: This condition can occur in children with cataracts due to uneven visual input to the brain, causing misalignment of the eyes. Treatment may involve glasses, patching, eye exercises, or surgery to align the eyes properly.[117]
- Inflammation and infection: Postoperative inflammation and infection (endophthalmitis) are serious complications that can occur after cataract surgery. Prophylactic antibiotics and anti-inflammatory medications are used postoperatively. Prompt recognition and treatment of infections are crucial to prevent severe outcomes.[118]
- Retinal detachment: Retinal detachment is a rare but serious complication where the retina separates from the back of the eye, leading to vision loss. Immediate surgical intervention is required to reattach the retina and restore vision.[119]
- Refractive errors: Postoperative refractive errors such as myopia (nearsightedness) or hyperopia (farsightedness) can occur, affecting visual clarity. Corrective lenses (glasses or contact lenses) are prescribed to address refractive errors and ensure optimal visual outcomes.[120]
- Phthisis bulbi: Phthisis bulbi is a condition where the eye becomes shrunken and nonfunctional due to severe damage or inflammation. Preventive measures include timely and effective management of complications. Enucleation (removal of the eye) may be considered in severe cases.[121]
- Corneal opacification: Corneal opacification can occur due to chronic inflammation or infection, leading to reduced corneal transparency. Regular monitoring and managing underlying causes, such as infections and inflammation, are crucial. Corneal transplantation may be considered in severe cases.
- Other complications include cystoid macular edema, retinal detachment, endophthalmitis, heterochromia, corneal decompensation, bullous keratopathy, astigmatism, and postoperative refractive surprise may also be seen.[122]
Monitoring and managing complications associated with pediatric cataracts are critical to achieving the best possible visual outcomes. A multidisciplinary approach involving regular follow-ups, prompt intervention, and comprehensive care is essential to address these complications effectively and enhance the quality of life for affected children.
Postoperative and Rehabilitation Care
Early visual rehabilitation of children with pediatric cataracts is as crucial as early diagnosis and treatment. Rehabilitation reduces the incidence of amblyopia, strabismus, and poor fusion.
Some options for visual rehabilitation in these children are spectacles, contact lenses, intraocular lenses, epikeratophakia, low vision aids, and amblyopia therapy. Parents should be counseled about the need for regular follow-up and routine refraction due to frequent refractive changes as the eyeball grows with age.
Children less than 2 years old need aphakic glasses or contact lenses for visual rehabilitation after cataract surgery till the secondary IOL implantation is planned. Aphakic glasses are usually used for bilateral aphakic patients. Due to improved surgical technique and an increased number of primary IOL implantations, the use of aphakic glasses has been reduced. Aphakia treatment aims to achieve the correct optical correction after cataract extraction is done to permit the visual system's early symmetrical stimulation in the critical development period.[123]
- Spectacles are the earliest form of aphakia treatment. Aphakic glasses with bifocal addition remain the primary mode of visual rehabilitation in bilateral aphakic children, which is preferred due to their affordability, accessibility, and lack of ocular discomfort or direct contact with the eye's surface. Notable disadvantages include the weight, large size, restriction of the field of vision, increasing nystagmus, jack-in-the-box phenomenon, pin cushion phenomenon, and ring scotoma. In cases of unilateral cataracts, anisometropia, and anisokenia lead to diplopia and amblyopia, which further cause permanent suppression and anomalous retinal correspondence.
- Contact lenses are the best optical device for correcting postoperative aphakia. These lenses should be worn during the daytime and removed during the night. Contact lenses are superior to spectacles in both unilateral and bilateral cases. Contact lenses offer a larger visual field, decreased glare and spherical aberrations, and better binocularity and cosmetic appearance. Intolerability, cost, and noncompliance pose significant problems for children and their parents. Chronic eye disease and ocular surface disorders are notable contraindications to its use. Three types of contact lenses are available for pediatric patients: polymethylmethacrylate (PMMA) or rigid gas permeable, silicone elastomer, and hydrogel lenses. The ideal contact lenses are the extended type. However, extended-type lenses' complications like giant papillary conjunctivitis, neovascularisation, abrasions, and infective keratitis make daily-use lenses preferred.
Children older than age 2 years with primary IOL implantation also require adequate visual rehabilitation postsurgery. Spectacles or contact lenses may be prescribed after refraction.
Amblyopia Treatment
Deprivational amblyopia is more prevalent in pediatric cataracts, especially within the critical developmental period of 2 months, leading to unequal visual input in the eyes. Consequently, unilateral cataracts induce denser amblyopia than bilateral ones, necessitating earlier surgical intervention. Amblyopia therapy, essential for visual rehabilitation, should commence promptly, with occlusion therapy being the primary treatment. However, ensuring compliance with therapy remains a significant challenge. Thus, proper counseling of parents is crucial for achieving successful visual outcomes. Patients undergo reevaluation every 2 weeks to monitor visual improvement or amblyopia reversal.[124]
Low Vision Aids
These devices enable children to improve their visual performance and help them reach their full capacity. Many devices are available according to the patient's age and parents' affordability. Convex lenses are used in various forms as primary optical assistance. Other devices are standard additional bifocals, hand magnifiers, stand magnifiers, electronic magnifiers, telescopes, and computer-adaptive closed-circuit televisions.[125]
Immediate Postoperative Care
- Monitoring for complications: Closely monitoring for any immediate postoperative complications such as infection, increased intraocular pressure, and inflammation is essential.
- Medications: Administer prescribed medications, including antibiotics and anti-inflammatory drops, to prevent infection and control inflammation.
- Pain management: Ensure appropriate pain management to keep the child comfortable following surgery.[126]
Follow-Up Visits
- Regular Check-Ups: Schedule regular follow-up appointments with the pediatric ophthalmologist to monitor healing and detect any complications early.
- Assessment of visual acuity: Regular assessments of the child's visual acuity to ensure the success of the surgery and to plan for any necessary interventions.
- Intraocular pressure monitoring: Check for signs of glaucoma, a potential postoperative complication, through regular intraocular pressure measurements.[127]
Secondary Interventions:
- PCO: Be vigilant for the development of PCO, which may require treatment with neodymium laser capsulotomy.
- Strabismus management: Address any issues with eye alignment (strabismus) that may develop or persist after cataract surgery.
Rehabilitation Care
Postoperative rehabilitation is essential for good outcomes. Rehabilitation comprises the crucial interventions discussed below.
Visual Rehabilitation
- Corrective lenses: Prescribe appropriate corrective lenses (glasses or contact lenses) to address any residual refractive errors and support optimal visual development.
- Amblyopia treatment: Implement amblyopia therapy, such as patching the stronger eye, to strengthen the weaker eye and improve binocular vision.[128]
Occupational Therapy
- Visual skills training: Engage the child in visual skills training to help them adapt to changes in vision and improve their functional use of vision in daily activities.
- Developmental support: Provide support for any developmental delays or challenges that may arise due to visual impairment.[129]
Parental Education and Support
- Postoperative care instructions: Educate parents on how to care for their child postsurgery, including administering eye drops and recognizing signs of complications.
- Long-term management: Inform parents about the importance of long-term follow-up and adherence to prescribed treatments and therapies to ensure the best possible visual outcomes.
- Emotional and psychological support: Provide access to counseling and support groups to help families cope with the emotional and psychological aspects of managing pediatric cataracts.[130]
School and Social Integration
- Educational support: Work with schools to ensure the child receives appropriate accommodations and support to facilitate learning and participation in school activities.
- Social skills development: Encourage activities that help the child develop social skills and interact with peers, enhancing overall quality of life.[131]
Effective postoperative and rehabilitation care for pediatric cataracts involves a comprehensive approach that includes regular monitoring, timely interventions, visual rehabilitation, and extensive support for both the child and their family. By addressing both the medical and developmental needs, healthcare providers can significantly improve visual outcomes and overall quality of life for children affected by pediatric cataracts.
Consultations
A pediatric cataract can manifest or be associated with a systemic disease involving other organ systems, such as the cardiac, renal, or central nervous systems. A pediatric consultation to rule out any other systemic comorbidities and gross congenital anomalies is a must. Moreover, since general anesthesia is required for children before surgery, a thorough preanesthetic evaluation is required to avoid untoward anesthetic complications on the table. Effective management of pediatric cataracts often requires a multidisciplinary approach involving consultations with various healthcare professionals to ensure comprehensive care.
- Pediatric ophthalmologist: A pediatric ophthalmologist is essential for diagnosing, performing surgery, and managing postoperative care for pediatric cataracts. These providers' expertise in specialized surgical techniques for children ensures proper visual rehabilitation. Consultation with a pediatric ophthalmologist should occur promptly upon suspicion or diagnosis of pediatric cataracts to plan timely surgical intervention and prevent long-term visual impairment.
- Pediatrician: The pediatrician is crucial to the child's overall health management, including identifying any underlying systemic conditions that may contribute to cataract formation. Pediatricians coordinate with specialists to manage comorbidities and provide comprehensive care. Regular pediatric check-ups should include screenings for eye health. Pediatricians should refer patients to a pediatric ophthalmologist if any signs of cataracts are observed.
- Genetic counselor: If cataracts are suspected to be hereditary, a genetic counselor can provide valuable information regarding the risk of recurrence in future pregnancies and offer support for genetic testing. Consultation with a genetic counselor should be considered when a family history of cataracts or genetic conditions is suspected.[132]
- Anesthesiologist: Given the need for surgical intervention in many cases of pediatric cataracts, an anesthesiologist with experience in pediatric cases is essential to ensure safe anesthesia management during cataract surgery. Anesthesia consultation should occur before surgery to assess the child's health and plan for safe anesthesia administration during the procedure.[133]
- Optometrist: Optometrists assist in the postoperative care of children with cataracts, including prescribing corrective lenses and monitoring visual development. These specialists are vital to ongoing visual rehabilitation. Regular consultations with an optometrist should be scheduled after surgery to ensure proper visual correction and monitor for any vision changes.[134]
- Occupational therapist: Occupational therapists can provide support for visual rehabilitation, helping children adapt to any changes in vision and improving their functional abilities in daily activities. Referral to an occupational therapist may be beneficial after surgery, particularly for children with significant visual impairment.[135]
- Social worker or psychologist: Providing emotional and psychological support to the child and family is crucial, especially if cataracts significantly impact the child's quality of life. Social workers or psychologists can offer counseling and connect families with support resources. Consultation should be considered early in the treatment process to provide comprehensive support for the family. An interprofessional approach involving timely consultations with various healthcare professionals is critical for effectively managing pediatric cataracts. Interprofessional care ensures that all aspects of the child’s health and well-being are addressed, leading to better visual and overall outcomes.
Managing pediatric cataracts demands a comprehensive approach that extends beyond ophthalmological care. Collaboration with healthcare professionals such as pediatricians, genetic counselors, anesthesiologists, optometrists, occupational therapists, and social workers or psychologists ensures holistic care tailored to the child's needs. Leveraging the expertise of this interprofessional team results in the effective management of pediatric cataracts, leading to improved visual and overall outcomes for affected children and their families.
Deterrence and Patient Education
Parents must be counseled about their child's condition and its etiology, treatment options, and visual prognosis. Parents should also be informed about the possible complications of the surgery and the need for regular, timely follow-ups with proper visual rehabilitation to give their child the best possible visual outcome.
Deterrence
Ensuring the health and well-being of both mother and child during pregnancy and infancy is paramount for preventing pediatric cataracts. Various deterrence measures can be implemented to safeguard against factors that may contribute to cataract formation in newborns and young children.
Prenatal and maternal healthcare
- Infection prevention: Encourage pregnant women to receive vaccinations against infections that can cause congenital cataracts, such as rubella. Advise them on avoiding exposure to infectious agents like Toxoplasma gondii and Cytomegalovirus.
- Nutritional support: Ensure pregnant women have access to proper nutrition, including essential vitamins and minerals that support fetal eye development, such as Vitamin A, folic acid, and ω-3 fatty acids.
- Regular prenatal checkups: Advocate for regular prenatal visits to monitor the health of both mother and fetus, allowing for early detection and management of any potential issues.[136]
Newborn screening and early detection
- Routine eye examinations: Implement routine eye screenings for newborns, including the red reflex test, to identify any early signs of cataracts. Early detection is crucial for timely intervention and better visual outcomes.
- Genetic counseling: Provide genetic counseling for families with a history of pediatric cataracts or related genetic conditions. Genetic counseling helps families understand their risks and prepare for early intervention if needed.[137]
Management of metabolic and systemic conditions
- Screening for metabolic disorders: Ensure newborns are screened for metabolic disorders such as galactosemia, diabetes, and hypocalcemia, which can lead to cataract formation. Early diagnosis and treatment can prevent cataract development.
- Regular monitoring: Children diagnosed with metabolic disorders should undergo regular eye examinations to monitor for the onset of cataracts and initiate treatment promptly.[138]
A comprehensive approach is necessary to mitigate the risk of pediatric cataracts. Pediatric cataracts may be minimized by focusing on infection prevention, nutritional support, regular prenatal checkups, newborn screening, genetic counseling, and management of metabolic and systemic conditions.
Patient Education
Educating parents and caregivers about pediatric cataracts is crucial for ensuring timely detection, treatment, and postoperative care. This educational approach encompasses several key areas, including understanding the nature of pediatric cataracts, emphasizing the importance of early treatment, and providing guidance on postoperative care and follow-up.
Raising awareness
- Understanding the condition: Educate parents and caregivers about the nature of pediatric cataracts, including potential causes, symptoms, and the importance of early detection. Use clear, nontechnical language to ensure comprehension.
- Importance of early treatment: Stress the significance of early intervention in preventing long-term visual impairment. Explain the potential benefits of timely surgical and medical treatments.
Postoperative care and follow-up
- Importance of follow-up appointments: Educate parents about the necessity of attending all follow-up appointments to monitor their child's recovery and detect any complications early.
- Adherence to medication regimens: Ensure parents understand the importance of adhering to prescribed medications, such as antibiotics or anti-inflammatory drugs, to prevent postoperative infections and inflammation.[139]
Visual rehabilitation
- Corrective lenses and patching: Inform parents about the need for corrective lenses (glasses or contact lenses) and occlusion therapy (patching) to improve visual outcomes and prevent amblyopia.
- Rehabilitation programs: Provide information on available visual rehabilitation programs and resources that can help enhance the child's visual development.
Support and resources
- Emotional and psychological support: Offer resources for emotional and psychological support to help families cope with the stress and challenges of managing pediatric cataracts.[140]
- Educational materials: Distribute educational materials such as brochures, videos, and online resources to reinforce understanding and provide ongoing support.
By focusing on prevention through prenatal care and early detection and providing thorough patient education, healthcare providers can greatly improve the management and outcomes of pediatric cataracts. This comprehensive approach ensures that children receive the best possible care, leading to better visual health and quality of life.[141]
Pearls and Other Issues
Pearls in Managing Pediatric Cataracts
Early detection and intervention are paramount in managing pediatric cataracts, with prompt diagnosis, ideally within the first few weeks of life. Routine red reflex testing should be conducted as part of newborn screening protocols to facilitate early identification. Customized treatment plans are essential, tailored to the cataract type and severity, child age, and associated conditions. This individualized approach ensures optimal surgical techniques and IOL selection to meet the child's specific needs. An interprofessional approach is fundamental, involving collaboration among pediatric ophthalmologists, pediatricians, anesthesiologists, optometrists, and support staff. This team can provide comprehensive care through coordinated efforts, addressing all aspects of pediatric cataract management to optimize outcomes and promote the child's visual health and well-being.
Disposition
Comprehensive preoperative evaluation is essential, including detailed ocular examination, assessment of visual acuity, and evaluation for any underlying systemic conditions. The timing of surgery is critical. For bilateral significant cataracts, surgery is recommended between 6 and 8 weeks of age, while for unilateral cataracts, it is typically between 4 and 6 weeks. Appropriate anesthesia and postoperative care are vital to minimize complications. Regular follow-up is necessary to monitor for complications such as glaucoma, PCO, and amblyopia. Visual rehabilitation, including using glasses, contact lenses, or patching, is crucial for optimal visual outcomes.[142]
Pitfalls
- Delayed diagnosis: Late presentation and diagnosis of pediatric cataracts can lead to irreversible visual impairment. Healthcare providers must be vigilant in screening for cataracts during routine check-ups.
- Inadequate postoperative management: Failure to manage postoperative complications effectively can compromise visual outcomes. Close monitoring for complications like increased intraocular pressure, uveitis, and secondary cataract formation is necessary.
- Noncompliance with follow-up: Ensuring that families understand the importance of adherence to follow-up visits and prescribed treatments, including patching and use of corrective lenses, is vital. Noncompliance can result in poor visual development and amblyopia.
- Genetic counseling: Genetic counseling can help families understand the risks and implications of hereditary cataracts. Prenatal screening for at-risk families may also be considered.
- Managing maternal infections: Preventing maternal infections during pregnancy, such as those caused by the TORCHES group, through vaccination and proper prenatal care can reduce the incidence of congenital cataracts.
- Addressing metabolic disorders: Early detection and management of metabolic disorders like galactosemia and diabetes can prevent the development of cataracts. Newborn screening programs play a critical role in identifying these conditions early.[143]
Additional Information
- Technological advances: Advances in surgical techniques and IOL technology have improved the outcomes of pediatric cataract surgeries. Techniques such as femtosecond laser-assisted cataract surgery and the use of newer IOL materials can enhance surgical precision and outcomes.
- Family education and support: Families must be educated about the condition, treatment options, and the importance of compliance with postoperative care. Psychological support can help families cope with the diagnosis and treatment process.
- Research and development: Ongoing research into pediatric cataracts' genetic and molecular basis is essential for developing new treatments and preventive strategies. Participation in clinical trials and registries can contribute to advancing knowledge in this field.[144]
Healthcare teams can ensure better management and outcomes for children with pediatric cataracts by focusing on these aspects, ultimately improving their quality of life and visual function.
Enhancing Healthcare Team Outcomes
Pediatric cataracts are generally present in primary care settings, with apprehensive parents bringing their child after noticing a white eye reflex (leukocoria), squinting, or nystagmus. Primary healthcare professionals play a vital role in the timely referral of such cases to centers with the expertise and capability to deal with the condition. Careful systemic and ocular examination to rule out other causes of leukocoria and any syndromic associations is essential before surgery. Timely diagnosis, management, and visual rehabilitation of a child with a pediatric cataract are crucial determinants of a favorable prognosis.[145]
An interprofessional team approach involving pediatric ophthalmology specialty-trained ophthalmic surgeons, pediatricians, anesthetists, optometrists, nurses, and informed and motivated parents helps bring about the best possible visual outcome. [Level 5]
Skills and Strategy
Effective management of pediatric cataracts requires a team with specialized skills and a strategic approach. Physicians, particularly pediatric ophthalmologists, need expertise in diagnosing and treating congenital and developmental cataracts. Advanced practitioners, including optometrists and pediatricians, play crucial roles in early detection and referral. Nurses must be skilled in preoperative and postoperative care, ensuring that children receive the necessary support throughout their treatment journey. Pharmacists provide valuable guidance on medication management, especially concerning any antifungal or antibiotic treatments used. A well-coordinated strategy that includes timely intervention and follow-up care is essential to prevent long-term visual impairment.
Ethics and Responsibilities
Ethical practice is vital for managing pediatric cataracts. Healthcare professionals must ensure informed consent is obtained from the parents or guardians, explaining the risks, benefits, and alternatives to treatment. Providers must respect the family's preferences and cultural beliefs while making decisions in the child's best interest. Responsibilities include maintaining patient confidentiality, providing equitable care, and being vigilant about potential ethical dilemmas, such as deciding the timing of surgical interventions or managing cases with poor prognoses.[146]
Interprofessional Communication
Clear and effective communication among healthcare team members is critical. Regular team meetings and the use of standardized communication tools, such as Situation-Background-Assessment-Recommendation, can help ensure that everyone is on the same page regarding the patient's care plan. Digital tools like electronic health records facilitate real-time information sharing and collaboration among physicians, nurses, pharmacists, and other specialists involved in the child's care.[147]
Care Coordination
Care coordination is essential to ensure comprehensive and continuous care for children with pediatric cataracts. Care coordination involves collaboration among healthcare providers to create and implement individualized care plans. Coordinators or case managers can help navigate the healthcare system, schedule necessary appointments, and complete follow-ups. This holistic approach helps address all aspects of the child's health, including managing comorbidities and ensuring adherence to treatment regimens.[148]
Patient-Centered Care
Patient-centered care focuses on the unique needs of each child and their family. Patient-centered care involves engaging families in decision-making, providing education about the condition and treatment options, and offering support resources. This element is crucial to ensure that care is tailored to the child's developmental stage and that the family is supported emotionally and socially. This approach improves compliance with treatment plans and enhances overall satisfaction and outcomes.[149]
Outcomes and Patient Safety
Improving outcomes and ensuring patient safety are paramount, including minimizing the risk of surgical complications, preventing infections, and ensuring accurate and safe medication administration. Implementing evidence-based practices, such as using sharp-edged IOLs to reduce visual opacification, can significantly enhance outcomes. Regular training and adherence to safety protocols are essential to maintaining high standards of care.[150]
Team Performance
Enhancing team performance involves continuous education, regular performance evaluations, and fostering a culture of collaboration. Encouraging open communication and valuing each team member's input can lead to more effective teamwork. Utilizing performance metrics to track outcomes and identify areas for improvement can drive quality improvement initiatives. Providing opportunities for professional development ensures that all team members are up-to-date with the latest advancements in pediatric cataract management.
In summary, managing pediatric cataracts effectively requires a multidisciplinary approach that includes specialized skills, strategic planning, ethical practice, effective communication, coordinated care, patient-centered approaches, and continuous improvement in team performance. Healthcare teams can provide high-quality, safe, and effective care by focusing on these areas, leading to better outcomes for children with pediatric cataracts.[151]