Introduction
Gynecological sonography is an important diagnostic modality commonly used to evaluate emergent conditions and many common gynecological disorders. Knowledge of the anatomy and physiology of the female pelvis is an important prerequisite in obtaining and interpreting gynecological sonographic images.
Structure and Function
The female pelvis is made up of two hip bones, articulating with each other anteriorly at the pubic symphysis and posteriorly with the sacrum via sacroiliac joints bilaterally. Each hip bone, in turn, is composed of three bones: the ilium, the ischium, and the pubic bones (which completely fuse around puberty). The primary function of the pelvis is to transfer the weight of the upper body i
nto the lower limbs and protect and elevate pelvic and lower abdominal organs. The pelvic girdle is divided into the greater/false pelvis (containing lower abdominal viscera) and the lesser/true pelvis (containing pelvic organs). The junction between the two is defined by the pelvic inlet.
The pelvic inlet is bordered by pubic symphysis anteriorly, sacral ala posteriorly, and laterally it is outlined by the arcuate line of the ilium and the pectineal line of the superior pubic ramus. The female pelvis is wider, broader, oval-shaped with a greater diameter than the male pelvis, which is clearly adapted for childbirth.
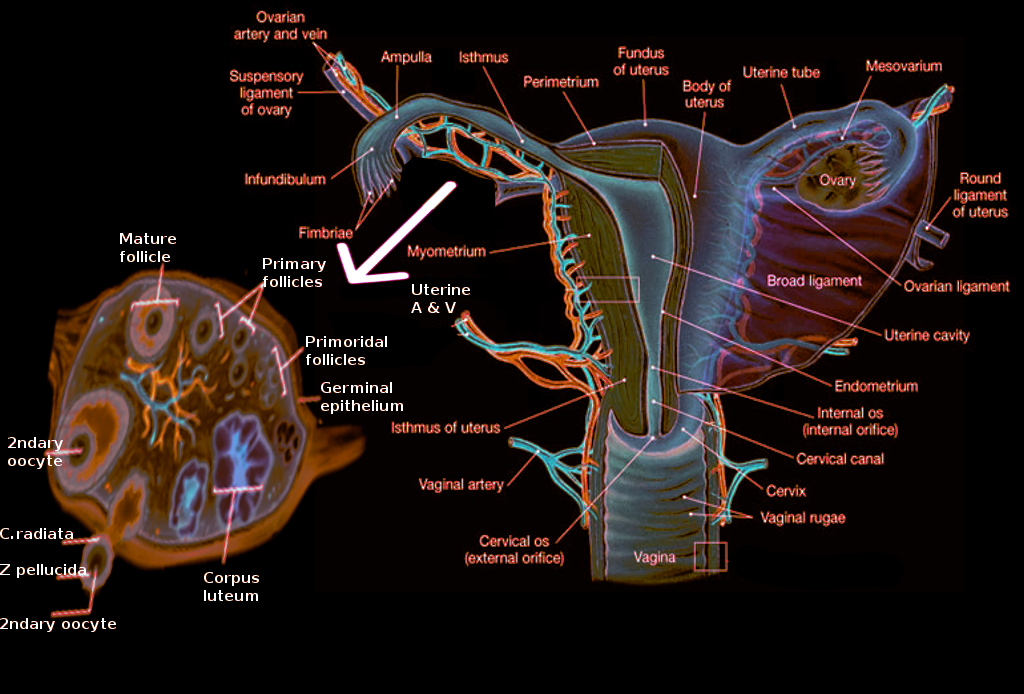
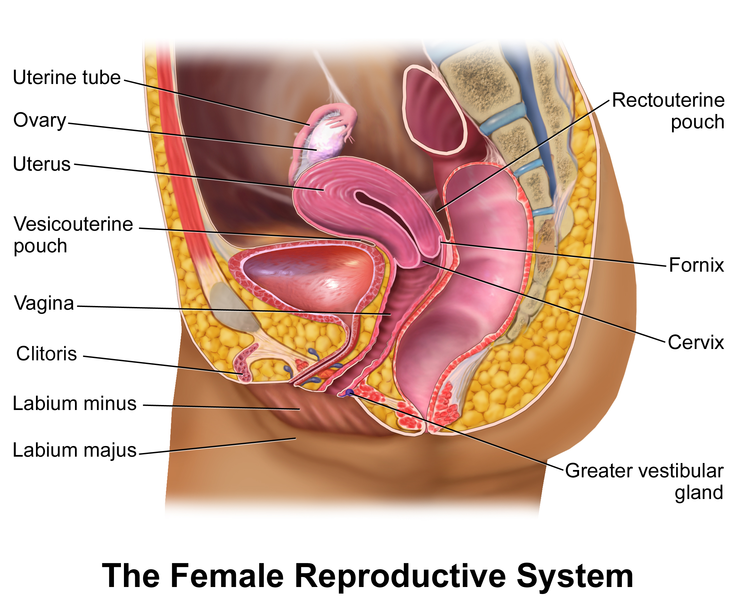
In females, pelvic organs include the reproductive organs such as the uterus, paired fallopian tubes, and ovaries, along with other organs such as the bladder, rectum, surrounded by ligaments, nerves, lymph nodes, and vasculature (see Image. The Female Reproductive System).[1] The uterus acts as a site for menstruation as well as protection and support for the growing fetus. The uterus is pear-shaped and positioned in the center of the pelvic cavity (behind the bladder and anterior to the rectum) and consists of four parts: the fundus, body, isthmus, and cervix. Its average length is approximately 8 cm, and its width is 5 cm.[2]
The body is the largest part of the uterus and is wider at the fundus and narrows down, creating the isthmus with its lowest section, the cervix. The cervix opens into the vagina. The uterine wall contains three layers. From inside out, they are the endometrium, myometrium, and perimetrium. The endometrium is the mucous lining of the uterus with basal and functional layers, while the myometrium is a layer of the smooth muscle undergoing hypertrophy during pregnancy. The functional layer of the endometrium changes during the menstrual cycle.[3]
The proliferative phase begins after menstruation and allows regeneration of the functional layer. After ovulation (in midcycle), under the influence of increased levels of progesterone secreted by the corpus luteum, the endometrium enters the secretory phase characterized by increased mucus production. It continues until menstruation occurs when the functional layer sheds and the cycle is repeated. The perimetrium is part of the peritoneum and is continuous with the abdominal peritoneal layer. The anatomical position of the uterus can be described as "anteverted" with respect to the vagina and "anteflexed" based on its relationship to the cervix. In the most common orientation, the uterus is "anteverted and anteflexed," which means that it's rotated and flexed forward towards the abdominal wall (see Image. Ultrasound Uterus Sagittal, Anteverted and Anteflexed Orientation).
Paired fallopian tubes connect to the uterus at the level of the fundus and extend laterally, opening into the abdominal cavity. The main function of the fallopian tubes is the transport of the ovum from laterally positioned ovaries into the uterus. It also acts as the main site of fertilization. From medial to lateral, fallopian tubes have the following parts: intramural (embedded within the wall of the uterus and the most narrow part), isthmus (extending from the uterus), followed by the ampulla (the widest section and the most common site of fertilization) opening into the infundibulum with finger-like projections called fimbriae.[3]
Fimbriae "extend" towards the ovary to "catch" the ovum. The ovaries are paired female gonads producing the female gametes (oocytes) as well as sex hormones, estrogen, and progesterone (see Image. Ovary Anatomy). Ovaries are attached to the broad ligament on the posterior part of the uterus by the mesovarium. The broad ligament provides support for the female reproductive tract. It is a sheet of peritoneum extending from lateral walls and folding over the uterus anteriorly and posteriorly. Ovaries undergo physiological changes with the menstrual cycle.
During the follicular phase, a few ovarian follicles proliferate, ultimately one of them developing into a dominant follicle (Graafian follicle), which can be up to 24 mm in size. During ovulation, the dominant follicle releases the ovum. The follicle then enters the luteal phase and develops into the corpus luteum (thick-walled cystic structure), producing progesterone and regressing in the absence of pregnancy.
Embryology
Embryonic development of the pelvis is complex, and it begins around day 28 of intra-embryonic life. Initially, after fertilization of the egg, blastula formation, and its implantation into the uterine wall, three germ layers are formed: the endoderm (the inner layer), the mesoderm (the middle layer), and the ectoderm (the outer layer). Subsequently, mesoderm differentiates into three components on each side of the midline: paraxial mesoderm (most medial), intermediate mesoderm, and lateral plates (most lateral). The paraxial mesoderm develops into the axial skeleton, muscles, tendons, cartilage, and dermis. Intermediate mesoderm gives rise to female reproductive organs and the urogenital system. Lateral plates develop into visceral and parietal pleura, appendicular skeleton (limbs, shoulder, and pelvic girdles), and blood vessels.
By approximately the third week of development, the paraxial mesoderm begins organizing into segments, or somitomeres in the head, and somites from occiput caudally. By the fifth week, there are a total of 42-44 pairs of somites arranged symmetrically on both sides of the neural tube. Ventral and medial wall cells of somites move more medially and surround the neural tube, eventually forming the vertebral bodies and ribs. Dorsal and lateral wall cells of somites develop into muscle precursor cells. Other cells of somites move beneath the ectoderm and form the dermis of the back. Muscle precursor cells create connective tissue called the mesenchyme.[4]
Towards the end of the fourth week of development, the lateral plate mesoderm protrudes through the lateral body wall, creating four limb buds covered by the ectoderm (the outer germ layer). The limbs grow in proximal to distal orientation. Intermediate mesoderm grows from cranial to caudal in paired structures, the mesonephric ducts with nephrogenic cords (and paramesonephric ducts). Even though sex is determined at fertilization, morphological characteristics start appearing only by the 7th week of intrauterine life. The gender of the developing fetus is determined primarily by the Y chromosome, which consists of testis-determining gene/factor, which is known as SRY (sex-determining region) on the short arm of the Y chromosome (Yp11).[5]
Initially, gonads appear as ridges on the nephrogenic cords. Primordial germ cells primarily originate in epiblast but migrate to endoderm cells within the yolk sac close to allantois approximately the 3rd week. Then migrate along the dorsal mesentery of the hindgut. Epithelial germ cells invade gonadal ridges causing proliferation and development of the primitive sex cords (medullary and cortical) at the 6th week of the development. It is important to note that if the germ cells fail to reach the ridges, there will be no development of gonads in the developing fetus. The cortical sex cords become the ovaries, while medullary sex cords eventually disintegrate. Mesonephric ducts degenerate in females in the absence of the Y chromosome. Paramesonephric ducts give rise to fallopian tubes (with its distal parts remaining open to the abdominal cavity with fimbriae), and the uterine cavity after distal portions of the bilateral paramesonephric ducts fuse around week 9. The tip of the fused ducts invades the urogenital sinus forming the sinovaginal bulb, which eventually cannulates, creating the distal part of the vagina by week 20. Therefore, the proximal part of the vagina (the fornices) is derived from the distal part of the fused paramesonephric ducts.
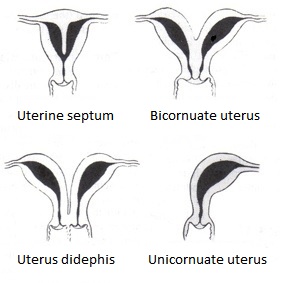
When paramesonephric ducts fail to fuse, a didelphic uterus is formed with paired uterine bodies and cervices. A relatively common condition is uterus bicornis, resulting from incomplete obliteration of the uterine septum after fusion of the paramesonephric ducts during embryological development. Septate uterus results from incomplete disintegration of the septum between 2 paramesonephric ducts (see Image. Bicornuate Uterus Malformation).[6][7]
The mesonephric duct (Wolffian duct) totally disappears in a female, and its remnant is the Gartner’s duct. Some of the components of the mesonephric duct remain what are seen as epoophoron & paroophoron. A tiny caudal portion of this Gartner’s duct is found in the wall of the uterus or vagina in the form of Gartner’s cysts.[8][9]
Blood Supply and Lymphatics
The abdominal aorta bifurcates into common iliac arteries, which, in turn, are divided into external and internal iliac arteries at the L5-S1 vertebral level. The internal iliac arteries are the major blood supply to the pelvis. They are coursing inferiorly into the lesser pelvis, medial to the external iliac veins, and obturator nerves. At the greater sciatic foramen, the internal iliac artery divides into anterior and posterior trunks. The anterior trunk consists of the following 8 branches in females: inferior gluteal, internal pudendal, middle rectal, uterine, vaginal, inferior vesicle, umbilical, and obturator. The posterior trunk had 3 branches: superior gluteal, lateral sacral, and iliolumbar. The uterus receives the majority of its blood supply from the uterine arteries.
Uterine arteries branch into arcuate arteries, which penetrate and supply the myometrium. However, the ovarian arteries also provide blood supply to the uterus, the fallopian tubes, and the ovaries. The paired ovarian arteries are aortic branches arising below the renal arteries. Ovaries receive their blood supply mainly from the paired ovarian arteries. However, a branch for the ovarian artery creates a direct anastomosis with the uterine artery; thus, ovaries receive a dual blood supply. Vaginal arteries supply the lower part of the female reproductive tract. Venous supply follows the arterial supply of the pelvic organs.[10] They begin as a complex network of very small veins around the body of the uterus, forming uterine venous plexus draining into uterine veins.[11]
In the ovaries, this network of veins is known as the pampiniform plexus of veins that drain into the ovarian veins. The ovarian vein drains into the left renal vein on the left side, but the inferior vena cava on the right. The fallopian tube is partly drained by the uterine venous plexus and partly by the pampiniform plexus.[12]
Lymphatic drainage of the pelvis is accomplished through a chain of lymph nodes named after the associated arteries: sacral, internal iliac, external iliac, and common iliac nodes. The vagina, cervix, and uterus drain into external and internal iliac nodes. Fallopian tubes and ovaries drain into the paraaortic lymph nodes.[13]
Nerves
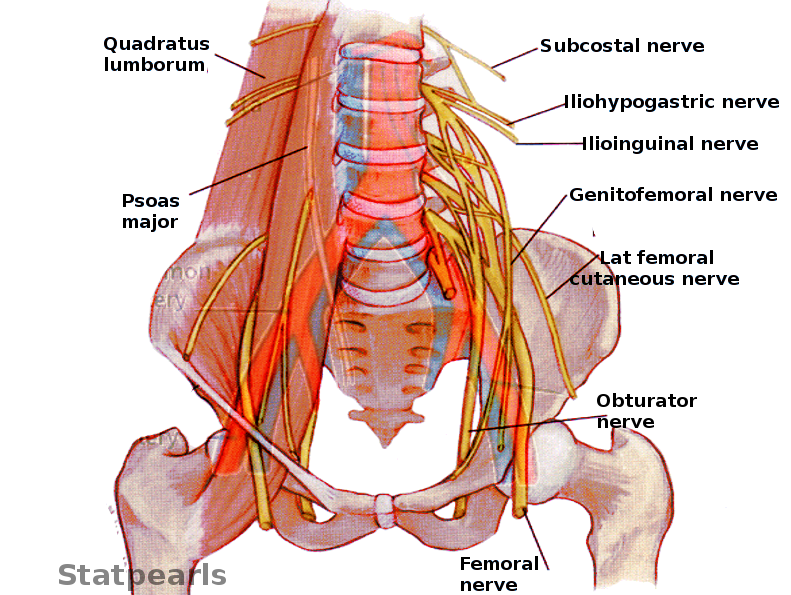
Innervation of the female pelvis is accomplished by visceral/autonomic and somatic nerve fibers. Sacral 2-4 roots provide parasympathetic fibers (pelvic splanchnic nerves) to the inferior hypogastric plexuses.[14] Superior hypogastric plexus, located anterior to the aorta just above its bifurcation, gives origin to the sympathetic fibers running distally along the iliac arteries (right and left hypogastric nerves), meeting pelvic splanchnic nerves at their respective inferior hypogastric plexuses. Thus, the inferior hypogastric plexuses provide parasympathetic and sympathetic innervation to the pelvic viscera (see Image. Pelvic Nerves).
The lumbar-sacral plexus is formed by nerves originating from L 4-5 and S 1-4 roots and lies, as a flat sheet, between the piriformis muscles and piriformis fascia bilaterally. The lumbar-sacral plexus innervates muscles of the pelvic walls, floor, external anal sphincter, and gluteal muscles and provides preganglionic fibers to pelvic splanchnic nerves. There are also some cutaneous branches supplying the back of the thigh and the gluteal region. The superior gluteal nerve (L4, L5, S1) leaves the pelvic via the greater sciatic foramen superior to the piriformis and innervates gluteus minimus, medius, and tensor fascia lata.
The inferior gluteal nerve (L4, L5, S1) leaves the pelvis via the greater sciatic foramen inferior to the piriformis and innervates the gluteus maximus. The posterior cutaneous nerve of the thigh (S1-3) runs deep below the gluteus maximus and down the back of the tight, providing innervation to the skin of the thigh, leg, and perineum. The pudendal nerve (S2-4) exits the pelvis via the greater sciatic foramen. It re-enters it via the lesser sciatic foramen, dividing into several branches: inferior rectal, perineal, and the dorsal nerve of the clitoris. These branches supply innervation to perineal skin, muscles, external anal sphincter, external urethral sphincter, the clitoris, and labia majora. The sciatic nerve (L4-5, S1-3) leaves the pelvic via the greater sciatic foramen and innervates muscles of the posterior thigh, entire leg, and foot and provides sensory innervation to the leg via its terminal branches.
Muscles
Lesser/true pelvic cavity is lined by obturator internus and piriformis muscles laterally. The piriformis muscle also creates the posterior wall. The inferior border of the pelvic cavity is the pelvic floor formed by levator ani and coccygeus muscles. The pelvic floor has 2 "gaps": urogenital hiatus and rectal hiatus. Three separate muscles form levator ani muscles: pubococcygeus, puborectalis, and iliococcygeus, symmetrically arranged about the midline.[15]
The pubococcygeus muscle is the main muscle of the levator ani providing support to the pubic viscera. Puborectalis muscle is a U-shaped muscle around the rectal hiatus. When contracted, it maintains fecal continence. This muscle is relaxed during defecation. Some fibers of the muscle loop around the urethra and vagina in females, assuring urinary continence when contracted. Iliococcygeus muscle elevates the pelvic floor. The coccygeus muscle is the most posterior muscle of the pelvic floor, adding to the muscles of the pelvic floor function of visceral support.
The perineal body is a fibromuscular structure in between the vagina and anal canal that is very important in support of the pelvic viscera. Any damage to the perineal body might (usually during vaginal delivery) lead to prolapse of the uterus and other pelvic organs.[16]
Physiologic Variants
When evaluating the female pelvis, physiological variants can be identified. Arterial supply may demonstrate some variability. Ovarian arteries may arise from renal arteries (and not the aorta itself) in 20% of cases.[17] Although in most females uterus is anteverted and anteflexed, it can also be retroverted or retroflexed. Although not physiologically important, the retroverted uterus is more likely to prolapse through the cervix into the vagina.[18] The arcuate uterus (with its fundus dipping into the uterine cavity) is considered a physiological variant since it does not have an increased risk of infertility.
Surgical Considerations
Hysterectomy is a common gynecological surgical procedure. When clamping uterine arteries, particular attention must be paid not to damage ureters given their close anatomical relationship. Uterine arteries travel in the lateral to the medial direction at the level of the cervix. Ureters lie 1.5 to 3 cm away from the uterine wall, so attention must be paid to the level where the uterine arteries meet the uterus. At this intersection of the uterine arteries and ureters, ureters lie under the arteries, so attention must be paid not to damage the ureters when clamping the arteries.[19]
Clinical Significance
The standard sonographic examination of the female pelvis includes the transabdominal approach and transvaginal approach with additional color or doppler ultrasonography if indicated. A high-frequency transducer (curvilinear probe) is used for transabdominal examination due to its ability to provide high spatial resolution. The vaginal transducer is also using higher frequencies and is used for transvaginal examination. However, its penetration is limited to approximately 12 cm, so a transabdominal examination is usually necessary to provide a complete evaluation of the pelvic organs. Adequate images can be obtained with or without a distended bladder, although it may be beneficial in pediatric patients.[20]
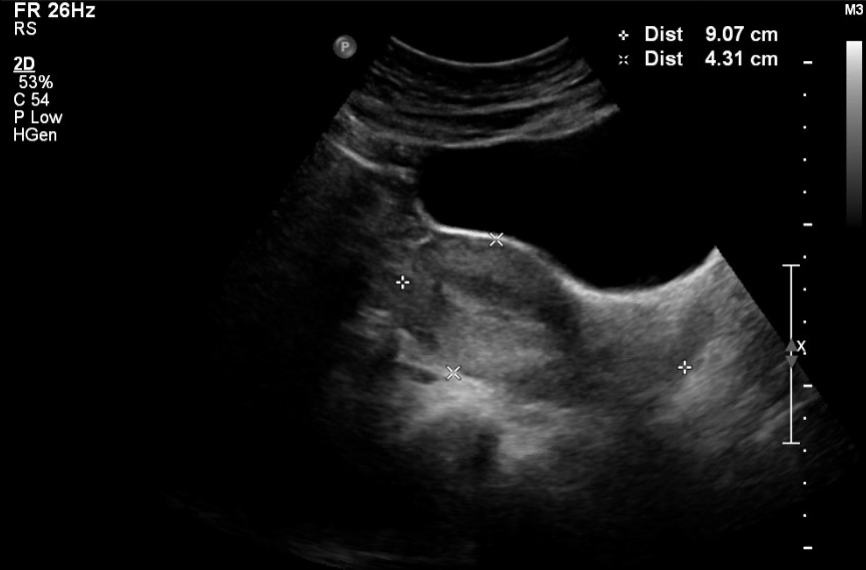
Evaluation of the uterus includes sagittal and transverse views. Typically, the transvaginal examination is performed first. The typical orientation of the uterus is anteverted and anteflexed so that other orientations can be noted. The length and height of the uterus are measured in the midsagittal plane. The uterine length has to be measured in the sagittal approach. The average uterine length is 7.3 to 9.2 cm in premenopausal women and 5.6-6.7 cm in postmenopausal women as myometrium atrophies with menopause.[21] The width is measured at the maximum width noted during the examination. The volume of the uterus can be calculated using the formula: length (not including the cervix) x height x width x 0.5233.[1]
Myometrium is homogeneous and echogenic, but sometimes arcuate vessels can be identified in the outer myometrium (which can have calcifications in older patients). The following abnormalities can be found within the myometrium: fibroids and adenomyosis (presence of abnormal endometrial tissue). Fibroids are common and are classified as intramural, submucosal, or subserosal. The endometrium should be measured at its widest point, from the outer border of the anterior endometrium to the outer border of the posterior endometrium, in sagittal view, not including any intrauterine fluid, if present. The endometrial appearance and thickness vary based on the phase of the menstrual cycle. During the proliferative phase, the endometrium has a "trilaminar" appearance and is typically < 8 mm. In the secretory phase, the endometrium appears more echogenic due to increased mucin production by the endometrial glands and is usually < 16 mm. During menstruation, blood and tissue can be found within the uterine cavity. Endometrial thickness of greater than 5 mm in postmenopausal women always warrants further investigation.[22]
Any cystic structures, irregularities, masses (frequently polyps), changes in echogenicity, or presence of an IUD can be noted. Cervix is measured from the internal oz to the external oz. Nabothian cysts can be appreciated as small cystic anechoic areas, which are benign in nature. Fibroids of the cervix have a similar appearance to the uterine fibroids. Early cervical cancer can not be visualized with sonography.
Evaluation of the adnexa and ovaries is best performed with the transvaginal approach. Ovaries must be imaged and measured in two orthogonal planes. Fallopian tubes (when normal) are not typically seen unless there is fluid in the cul-de-sac or hydrosalpinx. Hydrosalpinx results from distal fallopian tube obstruction, which is most commonly due to pelvic inflammatory disease. An ovary is an oval structure and can be identified in females of reproductive age by the presence of multiple follicles. With menopause, ovaries atrophy and become more challenging to identify by sonography. The mean ovarian volume in menstruating females is 9.8 cc, and in postmenopausal women, it's 5.8 cc.[1] Simple ovarian cysts are common and benign and must be differentiated from hemorrhagic cysts and ovarian masses.[23]
Ovarian torsion is twisting of the ovary on its pedicle, interrupting its blood supply, and represents a gynecological emergency. A torsed ovary appears enlarged and edematous. Commonly, an associated tumor or cyst may be found, which predisposes the patient to ovarian torsion. Sonographic assessment of the adnexa allows visualization of the uterine and ovarian arteries, which can be highlighted with color Doppler and show decreased flow when ovarian torsion is present. However, Doppler color may still be present even in cases of ovarian torsion due to the dual arterial supply of the ovaries.
Cul-de-sac (or the pouch of Douglas) may contain a small amount of free fluid in the space between the posterior wall of the uterus and the rectum. It is a frequent finding, present in up to 40% of women.[20] However, in the right clinical setting, free fluid in the pelvis may represent blood due to a ruptured ectopic pregnancy (with its most common location being the ampulla of the fallopian tube), warranting an emergent obstetric surgical intervention.