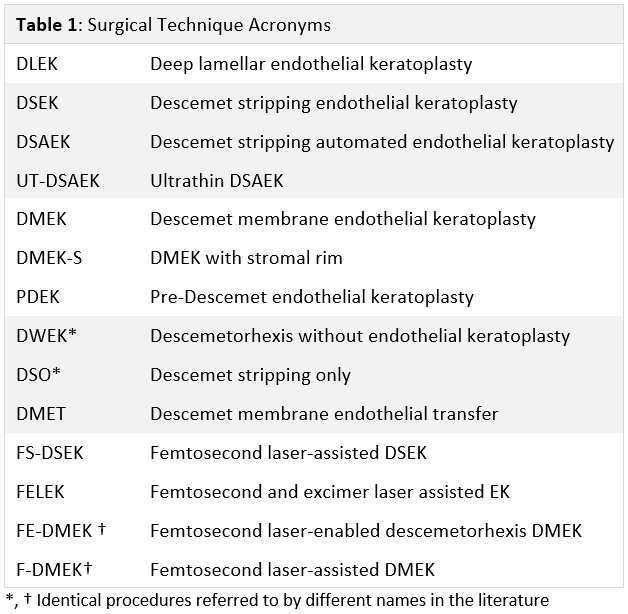
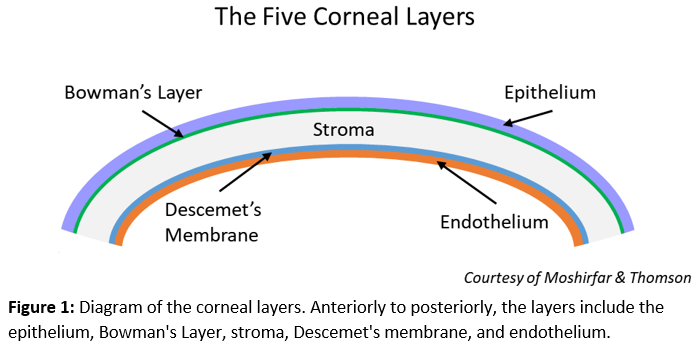
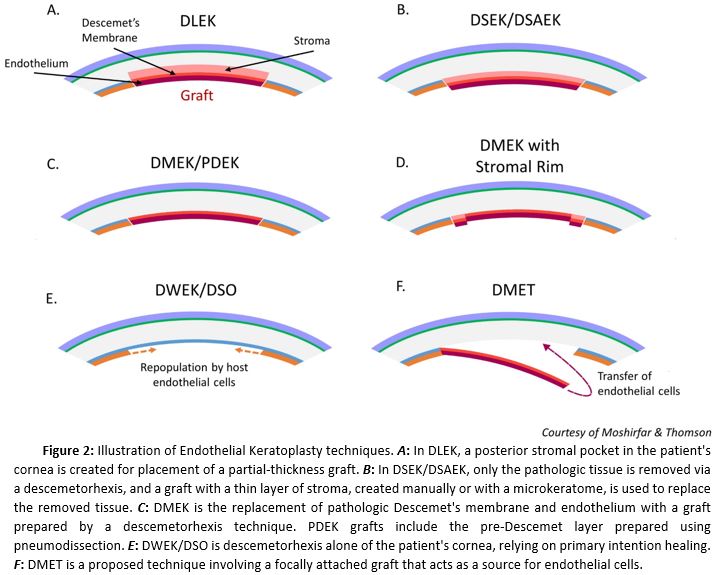
[1]
Price MO, Gupta P, Lass J, Price FW Jr. EK (DLEK, DSEK, DMEK): New Frontier in Cornea Surgery. Annual review of vision science. 2017 Sep 15:3():69-90. doi: 10.1146/annurev-vision-102016-061400. Epub 2017 Jul 11
[PubMed PMID: 28697678]
[2]
Güell JL, El Husseiny MA, Manero F, Gris O, Elies D. Historical Review and Update of Surgical Treatment for Corneal Endothelial Diseases. Ophthalmology and therapy. 2014 Dec:3(1-2):1-15. doi: 10.1007/s40123-014-0022-y. Epub 2014 Feb 18
[PubMed PMID: 25134494]
[3]
Moshirfar M, Ding Y, Shah TJ. A Historical Perspective on Treatment of Fuchs' Endothelial Dystrophy: We have Come a Long Way. Journal of ophthalmic & vision research. 2018 Jul-Sep:13(3):339-343. doi: 10.4103/jovr.jovr_94_18. Epub
[PubMed PMID: 30090191]
Level 3 (low-level) evidence
[4]
Zhang J, Patel DV, McGhee CNJ. The Rapid Transformation of Transplantation for Corneal Endothelial Diseases: An Evolution From Penetrating to Lamellar to Cellular Transplants. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2019 Nov-Dec:8(6):441-447. doi: 10.1097/APO.0000000000000265. Epub
[PubMed PMID: 31789646]
[6]
Barraquer JI. Lamellar keratoplasty. (Special techniques). Annals of ophthalmology. 1972 Jun:4(6):437-69
[PubMed PMID: 4555325]
[7]
Melles GR,Eggink FA,Lander F,Pels E,Rietveld FJ,Beekhuis WH,Binder PS, A surgical technique for posterior lamellar keratoplasty. Cornea. 1998 Nov;
[PubMed PMID: 9820943]
[8]
Melles GR, Lander F, van Dooren BT, Pels E, Beekhuis WH. Preliminary clinical results of posterior lamellar keratoplasty through a sclerocorneal pocket incision. Ophthalmology. 2000 Oct:107(10):1850-6; discussion 1857
[PubMed PMID: 11013184]
[9]
Terry MA, Ousley PJ. Deep lamellar endothelial keratoplasty in the first United States patients: early clinical results. Cornea. 2001 Apr:20(3):239-43
[PubMed PMID: 11322409]
[10]
Price FW Jr, Price MO. Descemet's stripping with endothelial keratoplasty in 50 eyes: a refractive neutral corneal transplant. Journal of refractive surgery (Thorofare, N.J. : 1995). 2005 Jul-Aug:21(4):339-45
[PubMed PMID: 16128330]
[11]
Melles GR, Wijdh RH, Nieuwendaal CP. A technique to excise the descemet membrane from a recipient cornea (descemetorhexis). Cornea. 2004 Apr:23(3):286-8
[PubMed PMID: 15084862]
[13]
Price FW Jr, Price MO. Descemet's stripping with endothelial keratoplasty in 200 eyes: Early challenges and techniques to enhance donor adherence. Journal of cataract and refractive surgery. 2006 Mar:32(3):411-8
[PubMed PMID: 16631048]
[14]
Price MO, Baig KM, Brubaker JW, Price FW Jr. Randomized, prospective comparison of precut vs surgeon-dissected grafts for descemet stripping automated endothelial keratoplasty. American journal of ophthalmology. 2008 Jul:146(1):36-41. doi: 10.1016/j.ajo.2008.02.024. Epub 2008 Apr 24
[PubMed PMID: 18439566]
Level 1 (high-level) evidence
[15]
Rose L, Briceño CA, Stark WJ, Gloria DG, Jun AS. Assessment of eye bank-prepared posterior lamellar corneal tissue for endothelial keratoplasty. Ophthalmology. 2008 Feb:115(2):279-86
[PubMed PMID: 17599413]
[16]
Palioura S, Colby K. Outcomes of Descemet Stripping Endothelial Keratoplasty Using Eye Bank-Prepared Preloaded Grafts. Cornea. 2017 Jan:36(1):21-25
[PubMed PMID: 27741016]
[17]
Neff KD, Biber JM, Holland EJ. Comparison of central corneal graft thickness to visual acuity outcomes in endothelial keratoplasty. Cornea. 2011 Apr:30(4):388-91. doi: 10.1097/ICO.0b013e3181f236c6. Epub
[PubMed PMID: 21045647]
[18]
Shinton AJ, Tsatsos M, Konstantopoulos A, Goverdhan S, Elsahn AF, Anderson DF, Hossain P. Impact of graft thickness on visual acuity after Descemet's stripping endothelial keratoplasty. The British journal of ophthalmology. 2012 Feb:96(2):246-9. doi: 10.1136/bjophthalmol-2011-300462. Epub 2011 Oct 25
[PubMed PMID: 22028474]
[19]
Feizi S, Javadi MA. Effect of Donor Graft Thickness on Clinical Outcomes after Descemet Stripping Automated Endothelial Keratoplasty. Journal of ophthalmic & vision research. 2019 Jan-Mar:14(1):18-26. doi: 10.4103/jovr.jovr_55_17. Epub
[PubMed PMID: 30820282]
Level 2 (mid-level) evidence
[20]
Busin M, Madi S, Santorum P, Scorcia V, Beltz J. Ultrathin descemet's stripping automated endothelial keratoplasty with the microkeratome double-pass technique: two-year outcomes. Ophthalmology. 2013 Jun:120(6):1186-94. doi: 10.1016/j.ophtha.2012.11.030. Epub 2013 Mar 1
[PubMed PMID: 23466268]
[21]
Dickman MM, Kruit PJ, Remeijer L, van Rooij J, Van der Lelij A, Wijdh RH, van den Biggelaar FJ, Berendschot TT, Nuijts RM. A Randomized Multicenter Clinical Trial of Ultrathin Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) versus DSAEK. Ophthalmology. 2016 Nov:123(11):2276-2284. doi: 10.1016/j.ophtha.2016.07.036. Epub
[PubMed PMID: 27659544]
Level 1 (high-level) evidence
[22]
Chamberlain W, Lin CC, Austin A, Schubach N, Clover J, McLeod SD, Porco TC, Lietman TM, Rose-Nussbaumer J. Descemet Endothelial Thickness Comparison Trial: A Randomized Trial Comparing Ultrathin Descemet Stripping Automated Endothelial Keratoplasty with Descemet Membrane Endothelial Keratoplasty. Ophthalmology. 2019 Jan:126(1):19-26. doi: 10.1016/j.ophtha.2018.05.019. Epub 2018 Jun 23
[PubMed PMID: 29945801]
Level 1 (high-level) evidence
[23]
Melles GR, Ong TS, Ververs B, van der Wees J. Preliminary clinical results of Descemet membrane endothelial keratoplasty. American journal of ophthalmology. 2008 Feb:145(2):222-227
[PubMed PMID: 18061137]
[24]
Agarwal A, Dua HS, Narang P, Kumar DA, Agarwal A, Jacob S, Agarwal A, Gupta A. Pre-Descemet's endothelial keratoplasty (PDEK). The British journal of ophthalmology. 2014 Sep:98(9):1181-5. doi: 10.1136/bjophthalmol-2013-304639. Epub 2014 Mar 21
[PubMed PMID: 24659352]
[25]
Price MO, Giebel AW, Fairchild KM, Price FW Jr. Descemet's membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009 Dec:116(12):2361-8. doi: 10.1016/j.ophtha.2009.07.010. Epub 2009 Oct 28
[PubMed PMID: 19875170]
Level 2 (mid-level) evidence
[26]
Trindade BLC, Eliazar GC. Descemet membrane endothelial keratoplasty (DMEK): an update on safety, efficacy and patient selection. Clinical ophthalmology (Auckland, N.Z.). 2019:13():1549-1557. doi: 10.2147/OPTH.S178473. Epub 2019 Aug 16
[PubMed PMID: 31496646]
[27]
Studeny P, Farkas A, Vokrojova M, Liskova P, Jirsova K. Descemet membrane endothelial keratoplasty with a stromal rim (DMEK-S). The British journal of ophthalmology. 2010 Jul:94(7):909-14. doi: 10.1136/bjo.2009.165134. Epub 2009 Oct 22
[PubMed PMID: 19850580]
[28]
Veldman PB, Dye PK, Holiman JD, Mayko ZM, Sáles CS, Straiko MD, Galloway JD, Terry MA. The S-stamp in Descemet Membrane Endothelial Keratoplasty Safely Eliminates Upside-down Graft Implantation. Ophthalmology. 2016 Jan:123(1):161-4. doi: 10.1016/j.ophtha.2015.08.044. Epub 2015 Oct 4
[PubMed PMID: 26439215]
[29]
Parekh M, Ruzza A, Ferrari S, Busin M, Ponzin D. Preloaded Tissues for Descemet Membrane Endothelial Keratoplasty. American journal of ophthalmology. 2016 Jun:166():120-125. doi: 10.1016/j.ajo.2016.03.048. Epub 2016 Apr 8
[PubMed PMID: 27066719]
[30]
Godinho JV, Mian SI. Update on Descemet membrane endothelial keratoplasty. Current opinion in ophthalmology. 2019 Jul:30(4):271-274. doi: 10.1097/ICU.0000000000000577. Epub
[PubMed PMID: 31045882]
Level 3 (low-level) evidence
[31]
Cheng YY, van den Berg TJ, Schouten JS, Pels E, Wijdh RJ, van Cleynenbreugel H, Eggink CA, Rijneveld WJ, Nuijts RM. Quality of vision after femtosecond laser-assisted descemet stripping endothelial keratoplasty and penetrating keratoplasty: a randomized, multicenter clinical trial. American journal of ophthalmology. 2011 Oct:152(4):556-566.e1. doi: 10.1016/j.ajo.2011.03.012. Epub 2011 Jun 17
[PubMed PMID: 21683332]
Level 2 (mid-level) evidence
[32]
Chen H, Tian L, Le Q, Zhao F, Zhao Y, Chen Y, Yang Y, Hong J, Xu J. Femtosecond laser-assisted Descemet's stripping endothelial keratoplasty: a prospective study of 6-month visual outcomes, corneal thickness and endothelial cell loss. International ophthalmology. 2020 Aug:40(8):2065-2075. doi: 10.1007/s10792-020-01383-8. Epub 2020 Apr 21
[PubMed PMID: 32318937]
[33]
Trinh L, Saubaméa B, Auclin F, Denoyer A, Lai-Kuen R, El Hamdaoui M, Labbé A, Despiau MC, Brignole-Baudouin F, Baudouin C. Femtosecond and excimer laser-assisted endothelial keratoplasty (FELEK): a new technique of endothelial transplantation. Journal francais d'ophtalmologie. 2014 Mar:37(3):211-9. doi: 10.1016/j.jfo.2013.07.009. Epub 2014 Feb 18
[PubMed PMID: 24559515]
[34]
Einan-Lifshitz A, Sorkin N, Boutin T, Showail M, Borovik A, Alobthani M, Chan CC, Rootman DS. Comparison of Femtosecond Laser-Enabled Descemetorhexis and Manual Descemetorhexis in Descemet Membrane Endothelial Keratoplasty. Cornea. 2017 Jul:36(7):767-770. doi: 10.1097/ICO.0000000000001217. Epub
[PubMed PMID: 28594697]
[35]
Pilger D, von Sonnleithner C, Bertelmann E, Maier AB, Joussen AM, Torun N. Exploring the precision of femtosecond laser-assisted descemetorhexis in Descemet membrane endothelial keratoplasty. BMJ open ophthalmology. 2018:3(1):e000148. doi: 10.1136/bmjophth-2018-000148. Epub 2018 Dec 27
[PubMed PMID: 30687781]
[36]
Sorkin N, Mednick Z, Einan-Lifshitz A, Trinh T, Santaella G, Telli A, Chan CC, Rootman DS. Three-Year Outcome Comparison Between Femtosecond Laser-Assisted and Manual Descemet Membrane Endothelial Keratoplasty. Cornea. 2019 Jul:38(7):812-816. doi: 10.1097/ICO.0000000000001956. Epub
[PubMed PMID: 30973405]
[37]
Gerber-Hollbach N, Parker J, Baydoun L, Liarakos VS, Ham L, Dapena I, Melles GR. Preliminary outcome of hemi-Descemet membrane endothelial keratoplasty for Fuchs endothelial dystrophy. The British journal of ophthalmology. 2016 Nov:100(11):1564-1568. doi: 10.1136/bjophthalmol-2015-307783. Epub 2016 Feb 2
[PubMed PMID: 26837507]
[38]
Birbal RS, Ni Dhubhghaill S, Baydoun L, Ham L, Bourgonje VJA, Dapena I, Oellerich S, Melles GRJ. Quarter-Descemet Membrane Endothelial Keratoplasty: One- to Two-Year Clinical Outcomes. Cornea. 2020 Mar:39(3):277-282. doi: 10.1097/ICO.0000000000002127. Epub
[PubMed PMID: 31490274]
Level 2 (mid-level) evidence
[39]
Bachmann B, Händel A, Siebelmann S, Matthaei M, Cursiefen C. Mini-Descemet Membrane Endothelial Keratoplasty for the Early Treatment of Acute Corneal Hydrops in Keratoconus. Cornea. 2019 Aug:38(8):1043-1048. doi: 10.1097/ICO.0000000000002001. Epub
[PubMed PMID: 31276462]
[40]
Garcerant D, Hirnschall N, Toalster N, Zhu M, Wen L, Moloney G. Descemet's stripping without endothelial keratoplasty. Current opinion in ophthalmology. 2019 Jul:30(4):275-285. doi: 10.1097/ICU.0000000000000579. Epub
[PubMed PMID: 31033737]
Level 3 (low-level) evidence
[41]
Van den Bogerd B, Dhubhghaill SN, Koppen C, Tassignon MJ, Zakaria N. A review of the evidence for in vivo corneal endothelial regeneration. Survey of ophthalmology. 2018 Mar-Apr:63(2):149-165. doi: 10.1016/j.survophthal.2017.07.004. Epub 2017 Aug 4
[PubMed PMID: 28782549]
Level 3 (low-level) evidence
[42]
Kaufman AR, Nosé RM, Pineda R 2nd. Descemetorhexis Without Endothelial Keratoplasty (DWEK): Proposal for Nomenclature Standardization. Cornea. 2018 Apr:37(4):e20-e21. doi: 10.1097/ICO.0000000000001528. Epub
[PubMed PMID: 29384812]
[43]
Macsai MS, Shiloach M. Use of Topical Rho Kinase Inhibitors in the Treatment of Fuchs Dystrophy After Descemet Stripping Only. Cornea. 2019 May:38(5):529-534. doi: 10.1097/ICO.0000000000001883. Epub
[PubMed PMID: 30720541]
[44]
Moloney G,Petsoglou C,Ball M,Kerdraon Y,Höllhumer R,Spiteri N,Beheregaray S,Hampson J,DʼSouza M,Devasahayam RN, Descemetorhexis Without Grafting for Fuchs Endothelial Dystrophy-Supplementation With Topical Ripasudil. Cornea. 2017 Jun;
[PubMed PMID: 28476048]
[45]
Huang MJ, Kane S, Dhaliwal DK. Descemetorhexis Without Endothelial Keratoplasty Versus DMEK for Treatment of Fuchs Endothelial Corneal Dystrophy. Cornea. 2018 Dec:37(12):1479-1483. doi: 10.1097/ICO.0000000000001742. Epub
[PubMed PMID: 30222714]
[46]
Dirisamer M, Yeh RY, van Dijk K, Ham L, Dapena I, Melles GR. Recipient endothelium may relate to corneal clearance in descemet membrane endothelial transfer. American journal of ophthalmology. 2012 Aug:154(2):290-296.e1. doi: 10.1016/j.ajo.2012.02.032. Epub 2012 May 23
[PubMed PMID: 22633346]
[47]
Dirisamer M, Ham L, Dapena I, van Dijk K, Melles GR. Descemet membrane endothelial transfer: "free-floating" donor Descemet implantation as a potential alternative to "keratoplasty". Cornea. 2012 Feb:31(2):194-7. doi: 10.1097/ICO.0b013e31821c9afc. Epub
[PubMed PMID: 22146548]
[48]
Birbal RS, Parker J, Dirisamer M, Janićijević A, Baydoun L, Dapena I, Melles GRJ. Descemet Membrane Endothelial Transfer: Ultimate Outcome. Cornea. 2018 Feb:37(2):141-144. doi: 10.1097/ICO.0000000000001395. Epub
[PubMed PMID: 28968295]
[49]
Sridhar MS. Anatomy of cornea and ocular surface. Indian journal of ophthalmology. 2018 Feb:66(2):190-194. doi: 10.4103/ijo.IJO_646_17. Epub
[PubMed PMID: 29380756]
[50]
Dua HS, Faraj LA, Said DG, Gray T, Lowe J. Human corneal anatomy redefined: a novel pre-Descemet's layer (Dua's layer). Ophthalmology. 2013 Sep:120(9):1778-85. doi: 10.1016/j.ophtha.2013.01.018. Epub 2013 May 25
[PubMed PMID: 23714320]
[51]
Barry PA, Petroll WM, Andrews PM, Cavanagh HD, Jester JV. The spatial organization of corneal endothelial cytoskeletal proteins and their relationship to the apical junctional complex. Investigative ophthalmology & visual science. 1995 May:36(6):1115-24
[PubMed PMID: 7730021]
[52]
Bourne WM. Biology of the corneal endothelium in health and disease. Eye (London, England). 2003 Nov:17(8):912-8
[PubMed PMID: 14631396]
[53]
Vedana G, Villarreal G Jr, Jun AS. Fuchs endothelial corneal dystrophy: current perspectives. Clinical ophthalmology (Auckland, N.Z.). 2016:10():321-30. doi: 10.2147/OPTH.S83467. Epub 2016 Feb 18
[PubMed PMID: 26937169]
Level 3 (low-level) evidence
[54]
Eghrari AO, Riazuddin SA, Gottsch JD. Fuchs Corneal Dystrophy. Progress in molecular biology and translational science. 2015:134():79-97. doi: 10.1016/bs.pmbts.2015.04.005. Epub 2015 Jul 15
[PubMed PMID: 26310151]
[55]
Pricopie S, Istrate S, Voinea L, Leasu C, Paun V, Radu C. Pseudophakic bullous keratopathy. Romanian journal of ophthalmology. 2017 Apr-Jun:61(2):90-94
[PubMed PMID: 29450379]
[56]
Walkden A, Au L. Iridocorneal endothelial syndrome: clinical perspectives. Clinical ophthalmology (Auckland, N.Z.). 2018:12():657-664. doi: 10.2147/OPTH.S143132. Epub 2018 Apr 9
[PubMed PMID: 29670326]
Level 3 (low-level) evidence
[57]
Silva L, Najafi A, Suwan Y, Teekhasaenee C, Ritch R. The iridocorneal endothelial syndrome. Survey of ophthalmology. 2018 Sep-Oct:63(5):665-676. doi: 10.1016/j.survophthal.2018.01.001. Epub 2018 Jan 11
[PubMed PMID: 29331589]
Level 3 (low-level) evidence
[58]
Lin ZN, Chen J, Cui HP. Characteristics of corneal dystrophies: a review from clinical, histological and genetic perspectives. International journal of ophthalmology. 2016:9(6):904-13. doi: 10.18240/ijo.2016.06.20. Epub 2016 Jun 18
[PubMed PMID: 27366696]
Level 3 (low-level) evidence
[59]
Feizi S. Corneal endothelial cell dysfunction: etiologies and management. Therapeutic advances in ophthalmology. 2018 Jan-Dec:10():2515841418815802. doi: 10.1177/2515841418815802. Epub 2018 Dec 7
[PubMed PMID: 30560230]
Level 3 (low-level) evidence
[60]
Ple-Plakon PA,Shtein RM, Trends in corneal transplantation: indications and techniques. Current opinion in ophthalmology. 2014 Jul;
[PubMed PMID: 24865170]
Level 3 (low-level) evidence
[61]
Pasari A, Price MO, Feng MT, Price FW Jr. Descemet Membrane Endothelial Keratoplasty for Failed Penetrating Keratoplasty: Visual Outcomes and Graft Survival. Cornea. 2019 Feb:38(2):151-156. doi: 10.1097/ICO.0000000000001763. Epub
[PubMed PMID: 30222720]
[62]
Tenkman LR, Price FW, Price MO. Descemet membrane endothelial keratoplasty donor preparation: navigating challenges and improving efficiency. Cornea. 2014 Mar:33(3):319-25. doi: 10.1097/ICO.0000000000000045. Epub
[PubMed PMID: 24452215]
[63]
Altaan SL, Gupta A, Sidney LE, Elalfy MS, Agarwal A, Dua HS. Endothelial cell loss following tissue harvesting by pneumodissection for endothelial keratoplasty: an ex vivo study. The British journal of ophthalmology. 2015 May:99(5):710-3. doi: 10.1136/bjophthalmol-2014-306560. Epub 2015 Mar 4
[PubMed PMID: 25740808]
[64]
Greiner MA,Rixen JJ,Wagoner MD,Schmidt GA,Stoeger CG,Straiko MD,Zimmerman MB,Kitzmann AS,Goins KM, Diabetes mellitus increases risk of unsuccessful graft preparation in Descemet membrane endothelial keratoplasty: a multicenter study. Cornea. 2014 Nov;
[PubMed PMID: 25222000]
Level 2 (mid-level) evidence
[65]
Terry MA, Aldave AJ, Szczotka-Flynn LB, Liang W, Ayala AR, Maguire MG, Croasdale C, Daoud YJ, Dunn SP, Hoover CK, Macsai MS, Mauger TF, Pramanik S, Rosenwasser GOD, Rose-Nussbaumer J, Stulting RD, Sugar A, Tu EY, Verdier DD, Yoo SH, Lass JH, Cornea Preservation Time Study Group. Donor, Recipient, and Operative Factors Associated with Graft Success in the Cornea Preservation Time Study. Ophthalmology. 2018 Nov:125(11):1700-1709. doi: 10.1016/j.ophtha.2018.08.002. Epub 2018 Aug 9
[PubMed PMID: 30098353]
[66]
Wakimasu K, Kitazawa K, Kayukawa K, Yokota I, Inatomi T, Hieda O, Sotozono C, Kinoshita S. Five-year follow-up outcomes after Descemet's stripping automated endothelial keratoplasty: a retrospective study. BMJ open ophthalmology. 2020:5(1):e000354. doi: 10.1136/bmjophth-2019-000354. Epub 2020 Jan 29
[PubMed PMID: 32154369]
Level 2 (mid-level) evidence
[67]
Chaurasia S, Price FW Jr, Gunderson L, Price MO. Descemet's membrane endothelial keratoplasty: clinical results of single versus triple procedures (combined with cataract surgery). Ophthalmology. 2014 Feb:121(2):454-8. doi: 10.1016/j.ophtha.2013.09.032. Epub 2013 Nov 16
[PubMed PMID: 24252821]
[68]
Terry MA, Shamie N, Chen ES, Phillips PM, Shah AK, Hoar KL, Friend DJ. Endothelial keratoplasty for Fuchs' dystrophy with cataract: complications and clinical results with the new triple procedure. Ophthalmology. 2009 Apr:116(4):631-9. doi: 10.1016/j.ophtha.2008.11.004. Epub 2009 Feb 8
[PubMed PMID: 19201480]
[69]
Burkhart ZN, Feng MT, Price FW Jr, Price MO. One-year outcomes in eyes remaining phakic after Descemet membrane endothelial keratoplasty. Journal of cataract and refractive surgery. 2014 Mar:40(3):430-4. doi: 10.1016/j.jcrs.2013.08.047. Epub 2014 Jan 11
[PubMed PMID: 24417895]
[70]
Price MO, Price DA, Fairchild KM, Price FW Jr. Rate and risk factors for cataract formation and extraction after Descemet stripping endothelial keratoplasty. The British journal of ophthalmology. 2010 Nov:94(11):1468-71. doi: 10.1136/bjo.2009.175174. Epub 2010 May 27
[PubMed PMID: 20508038]
[71]
Alkatan H, Al-Rajhi A, Al-Shehri A, Khairi A. Histopathological findings of failed grafts following Descemet's stripping automated endothelial keratoplasty (DSAEK). Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2012 Jan:26(1):79-85. doi: 10.1016/j.sjopt.2011.05.006. Epub 2011 Jun 1
[PubMed PMID: 23960973]
[72]
Khan SN, Shiakolas PS, Mootha VV. Descemet's Stripping Automated Endothelial Keratoplasty Tissue Insertion Devices. Journal of ophthalmic & vision research. 2015 Oct-Dec:10(4):461-8. doi: 10.4103/2008-322X.176899. Epub
[PubMed PMID: 27051492]
[73]
Busin M, Bhatt PR, Scorcia V. A modified technique for descemet membrane stripping automated endothelial keratoplasty to minimize endothelial cell loss. Archives of ophthalmology (Chicago, Ill. : 1960). 2008 Aug:126(8):1133-7. doi: 10.1001/archopht.126.8.1133. Epub
[PubMed PMID: 18695109]
[74]
Bahar I, Kaiserman I, Sansanayudh W, Levinger E, Rootman DS. Busin Guide vs Forceps for the Insertion of the Donor Lenticule in Descemet Stripping Automated Endothelial Keratoplasty. American journal of ophthalmology. 2009 Feb:147(2):220-226.e1. doi: 10.1016/j.ajo.2008.08.029. Epub 2008 Oct 18
[PubMed PMID: 18930446]
[75]
Kruse FE, Laaser K, Cursiefen C, Heindl LM, Schlötzer-Schrehardt U, Riss S, Bachmann BO. A stepwise approach to donor preparation and insertion increases safety and outcome of Descemet membrane endothelial keratoplasty. Cornea. 2011 May:30(5):580-7
[PubMed PMID: 21598430]
[76]
Burkhart ZN, Feng MT, Price MO, Price FW. Handheld slit beam techniques to facilitate DMEK and DALK. Cornea. 2013 May:32(5):722-4. doi: 10.1097/ICO.0b013e31827797e7. Epub
[PubMed PMID: 23407317]
[77]
Saad A, Guilbert E, Grise-Dulac A, Sabatier P, Gatinel D. Intraoperative OCT-Assisted DMEK: 14 Consecutive Cases. Cornea. 2015 Jul:34(7):802-7. doi: 10.1097/ICO.0000000000000462. Epub
[PubMed PMID: 26002152]
Level 3 (low-level) evidence
[78]
Nahum Y, Galor O, Atar M, Bahar I, Livny E. Real-time intraoperative ultrasound biomicroscopy for determining graft orientation during Descemet's membrane endothelial keratoplasty. Acta ophthalmologica. 2021 Feb:99(1):e96-e100. doi: 10.1111/aos.14515. Epub 2020 Jun 24
[PubMed PMID: 32578923]
[79]
Livny E, Bahar I, Nahum Y. "Ghost DMEK" Technique: Circular Peripheral Staining of Descemet's Membrane Endothelial Keratoplasty Grafts. Cornea. 2019 Feb:38(2):252-255. doi: 10.1097/ICO.0000000000001816. Epub
[PubMed PMID: 30422864]
[80]
Gonzalez A, Price FW Jr, Price MO, Feng MT. Prevention and Management of Pupil Block After Descemet Membrane Endothelial Keratoplasty. Cornea. 2016 Nov:35(11):1391-1395
[PubMed PMID: 27560030]
[81]
von Marchtaler PV, Weller JM, Kruse FE, Tourtas T. Air Versus Sulfur Hexafluoride Gas Tamponade in Descemet Membrane Endothelial Keratoplasty: A Fellow Eye Comparison. Cornea. 2018 Jan:37(1):15-19. doi: 10.1097/ICO.0000000000001413. Epub
[PubMed PMID: 29040116]
[82]
Feng MT, Price MO, Miller JM, Price FW Jr. Air reinjection and endothelial cell density in Descemet membrane endothelial keratoplasty: five-year follow-up. Journal of cataract and refractive surgery. 2014 Jul:40(7):1116-21. doi: 10.1016/j.jcrs.2014.04.023. Epub
[PubMed PMID: 24957432]
[83]
Greenrod EB, Jones MN, Kaye S, Larkin DF, National Health Service Blood and Transplant Ocular Tissue Advisory Group and Contributing Ophthalmologists (Ocular Tissue Advisory Group Audit Study 16). Center and surgeon effect on outcomes of endothelial keratoplasty versus penetrating keratoplasty in the United Kingdom. American journal of ophthalmology. 2014 Nov:158(5):957-66. doi: 10.1016/j.ajo.2014.07.037. Epub 2014 Aug 1
[PubMed PMID: 25089353]
[84]
Deng SX, Lee WB, Hammersmith KM, Kuo AN, Li JY, Shen JF, Weikert MP, Shtein RM. Descemet Membrane Endothelial Keratoplasty: Safety and Outcomes: A Report by the American Academy of Ophthalmology. Ophthalmology. 2018 Feb:125(2):295-310. doi: 10.1016/j.ophtha.2017.08.015. Epub 2017 Sep 15
[PubMed PMID: 28923499]
[85]
Dunker SL, Veldman MHJ, Winkens B, van den Biggelaar FJHM, Nuijts RMMA, Kruit PJ, Dickman MM, Dutch Cornea Consortium. Real-World Outcomes of DMEK: A Prospective Dutch registry study. American journal of ophthalmology. 2021 Feb:222():218-225. doi: 10.1016/j.ajo.2020.06.023. Epub 2020 Jul 2
[PubMed PMID: 32621899]
[86]
Maier AK, Klamann MK, Torun N, Gonnermann J, Schroeter J, Joussen AM, Rieck P. Intraocular pressure elevation and post-DSEK glaucoma after Descemet`s stripping endothelial keratoplasty. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2013 Apr:251(4):1191-8. doi: 10.1007/s00417-012-2203-5. Epub 2012 Nov 20
[PubMed PMID: 23180233]
[87]
Maier AB, Pilger D, Gundlach E, Winterhalter S, Torun N. Long-term Results of Intraocular Pressure Elevation and Post-DMEK Glaucoma After Descemet Membrane Endothelial Keratoplasty. Cornea. 2021 Jan:40(1):26-32. doi: 10.1097/ICO.0000000000002363. Epub
[PubMed PMID: 32558736]
[88]
Price MO, Jordan CS, Moore G, Price FW Jr. Graft rejection episodes after Descemet stripping with endothelial keratoplasty: part two: the statistical analysis of probability and risk factors. The British journal of ophthalmology. 2009 Mar:93(3):391-5. doi: 10.1136/bjo.2008.140038. Epub 2008 Nov 19
[PubMed PMID: 19019938]
[89]
Price DA, Kelley M, Price FW Jr, Price MO. Five-Year Graft Survival of Descemet Membrane Endothelial Keratoplasty (EK) versus Descemet Stripping EK and the Effect of Donor Sex Matching. Ophthalmology. 2018 Oct:125(10):1508-1514. doi: 10.1016/j.ophtha.2018.03.050. Epub 2018 May 3
[PubMed PMID: 29731147]
[90]
Hos D, Matthaei M, Bock F, Maruyama K, Notara M, Clahsen T, Hou Y, Le VNH, Salabarria AC, Horstmann J, Bachmann BO, Cursiefen C. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Progress in retinal and eye research. 2019 Nov:73():100768. doi: 10.1016/j.preteyeres.2019.07.001. Epub 2019 Jul 3
[PubMed PMID: 31279005]
[91]
Myerscough J, Friehmann A, Bovone C, Busin M. Autologous Descemet Stripping Automated Endothelial Keratoplasty to Eliminate Endothelial Rejection in Eyes at High Risk. Cornea. 2020 May:39(5):666-668. doi: 10.1097/ICO.0000000000002184. Epub
[PubMed PMID: 31688200]
[92]
Hos D, Tuac O, Schaub F, Stanzel TP, Schrittenlocher S, Hellmich M, Bachmann BO, Cursiefen C. Incidence and Clinical Course of Immune Reactions after Descemet Membrane Endothelial Keratoplasty: Retrospective Analysis of 1000 Consecutive Eyes. Ophthalmology. 2017 Apr:124(4):512-518. doi: 10.1016/j.ophtha.2016.12.017. Epub 2017 Jan 13
[PubMed PMID: 28094043]
Level 2 (mid-level) evidence
[93]
Crews JW, Price MO, Lautert J, Feng MT, Price FW Jr. Intraoperative hyphema in Descemet membrane endothelial keratoplasty alone or combined with phacoemulsification. Journal of cataract and refractive surgery. 2018 Feb:44(2):198-201. doi: 10.1016/j.jcrs.2017.11.015. Epub 2018 Mar 7
[PubMed PMID: 29525615]
[94]
Stuart AJ, Romano V, Virgili G, Shortt AJ. Descemet's membrane endothelial keratoplasty (DMEK) versus Descemet's stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. The Cochrane database of systematic reviews. 2018 Jun 25:6(6):CD012097. doi: 10.1002/14651858.CD012097.pub2. Epub 2018 Jun 25
[PubMed PMID: 29940078]
Level 1 (high-level) evidence
[95]
Zhu L, Zha Y, Cai J, Zhang Y. Descemet stripping automated endothelial keratoplasty versus descemet membrane endothelial keratoplasty: a meta-analysis. International ophthalmology. 2018 Apr:38(2):897-905. doi: 10.1007/s10792-017-0533-3. Epub 2017 Apr 17
[PubMed PMID: 28417337]
Level 1 (high-level) evidence
[96]
Marques RE, Guerra PS, Sousa DC, Gonçalves AI, Quintas AM, Rodrigues W. DMEK versus DSAEK for Fuchs' endothelial dystrophy: A meta-analysis. European journal of ophthalmology. 2019 Jan:29(1):15-22. doi: 10.1177/1120672118757431. Epub 2018 Apr 16
[PubMed PMID: 29661044]
Level 1 (high-level) evidence
[97]
Hirabayashi KE, Chamberlain W, Rose-Nussbaumer J, Austin A, Stell L, Lin CC. Corneal Light Scatter After Ultrathin Descemet Stripping Automated Endothelial Keratoplasty Versus Descemet Membrane Endothelial Keratoplasty in Descemet Endothelial Thickness Comparison Trial: A Randomized Controlled Trial. Cornea. 2020 Jun:39(6):691-696. doi: 10.1097/ICO.0000000000002256. Epub
[PubMed PMID: 31939923]
Level 1 (high-level) evidence
[98]
Birbal RS, Ni Dhubhghaill S, Bourgonje VJA, Hanko J, Ham L, Jager MJ, Böhringer S, Oellerich S, Melles GRJ. Five-Year Graft Survival and Clinical Outcomes of 500 Consecutive Cases After Descemet Membrane Endothelial Keratoplasty. Cornea. 2020 Mar:39(3):290-297. doi: 10.1097/ICO.0000000000002120. Epub
[PubMed PMID: 31478948]
Level 2 (mid-level) evidence
[99]
Madi S, Leon P, Nahum Y, DʼAngelo S, Giannaccare G, Beltz J, Busin M. Five-Year Outcomes of Ultrathin Descemet Stripping Automated Endothelial Keratoplasty. Cornea. 2019 Sep:38(9):1192-1197. doi: 10.1097/ICO.0000000000001999. Epub
[PubMed PMID: 31246680]
[100]
Koo EH, Pineda R, Afshari N, Eghrari A. Learning Descemet Membrane Endothelial Keratoplasty: A Survey of U.S. Corneal Surgeons. Cornea. 2020 May:39(5):590-593. doi: 10.1097/ICO.0000000000002203. Epub
[PubMed PMID: 31724984]
Level 3 (low-level) evidence