Learning Outcome
- List the causes of Cushing disease.
- Describe the presentation of Cushing disease.
- Summarize the treatment of Cushing disease.
- Recall the nursing care plans for Cushing disease.

Cushing disease is a rare disorder characterized by increased adrenocorticotropic hormone (ACTH) production from the anterior pituitary, leading to excess cortisol release from the adrenal glands.[1] Most often, this caused by a pituitary adenoma or as the result of excess production of corticotropin-releasing hormone (CRH) from the hypothalamus. Symptoms include generalized weakness, high blood pressure, diabetes mellitus, menstrual disorders, or psychiatric changes.[1] Physical manifestations of excess cortisol levels include moon facies, buffalo hump, bruising, abdominal striae, obesity, facial plethora, and hirsutism.[2]
Cushing disease is a relatively rare disease. The average incidence of new cases is about 2.4 cases per million people per year. This disease often is diagnosed 3 to 6 years after the onset of the illness. The peak incidence of Cushing disease is in women between the ages of 50 and 60 years. The prevalence of hypertension and abnormalities of glucose metabolism are major predictors of morbidity and mortality in untreated cases of the disease. The mortality rate of Cushing disease is estimated to be about 10% to 11%.[2]
Pituitary adenomas are responsible for nearly 80% of the cases of Cushing disease.[2] Of note, Cushing syndrome refers to the general state of hypercortisolemia, which can be caused by various mechanisms, including exogenous steroid use, adrenal tumors, ectopic-ACTH production, or high estrogen levels. Cushing disease is specific to the endogenous production of ACTH that leads to secondary hypercortisolism.
Harvey Cushing first described this disease in 1912 after he was presented with a unique case in 1910. Cushing hypothesized that excess basophil pituitary cells were responsible for his patients presenting symptoms of obesity, amenorrhea, abnormal hair growth, underdevelopment of sexual characteristics, hydrocephalus, and cerebral tension.[2]
Cushing disease is the second most common cause of Cushing syndrome, the first cause being exogenous steroids. The average incidence of new cases is about 2.4 cases per million people per year. This disease often is diagnosed 3 to 6 years after the onset of the illness. The peak incidence of Cushing disease is in women between the ages of 50 and 60 years. The prevalence of hypertension and abnormalities of glucose metabolism are major predictors of morbidity and mortality in untreated disease cases. The mortality rate of Cushing disease is estimated to be about 10% to 11%.[2]
Patients with hypercortisolism present with weight gain (50%), hypertension, easy bruising, striae, acne, flushing, poor wound healing, lower limb edema, fatigue, impaired glucose tolerance, osteoporosis, hyperpigmentation of the skin, mood and memory changes, amenorrhea, hirsutism, decreased sexual drive, or frequent infections. Clinical manifestations vary widely among patients; thus, a high index of clinical suspicion must be maintained in order to make this diagnosis correctly.[3]
Although uncommon, large pituitary tumors (macroadenomas) also can present with mass effects on surrounding structures. These cases may present with decreased peripheral vision or headaches.[3]
On presentation, more than half of the patients with Cushing disease have a microadenoma with a diameter of less than 5 mm.[4] Of these, only 10% are large enough to cause a mass effect on the cerebral tissue to affect the structure of the sellar region.[4] Therefore, most cases of ACTH-secreting pituitary adenomas are found after suspicion of excess cortisol and androgen production.[5]
Biochemical diagnostic tests to confirm hypercortisolism include salivary and blood serum cortisol testing, 24-hour urinary-free cortisol testing, and low-dose overnight dexamethasone suppression testing.[6] The late-night or midnight salivary cortisol test recently has been gaining support due to its ease of administration.[7] This test measures free-circulating cortisol and has both a sensitivity and specificity of 95% to 98%.[7] The urinary-free cortisol test measures the excess cortisol excreted by the kidneys into the urine.[8] Results that are four times higher than normal cortisol levels are considered to be attributable to Cushing syndrome. This test needs to be repeated three times to exclude any normal periods of hypercortisolism.[6] The specificity of this test is 81%.[6] The high false-positive rate can be caused by pseudo-Cushingoid states, sleep apnea, polycystic ovary syndrome, familial glucocorticoid resistance, and hyperthyroidism. In low-dose dexamethasone suppression testing, dexamethasone 0.5 mg is administered by mouth at six-hour intervals for 48 hours.[8] The serum cortisol level is measured 6 hours after the last dose of dexamethasone is given. A cortisol level of less than 50 nmol/L is considered a normal response and rules out Cushing syndrome. The sensitivity and specificity of this test are 100% and 88%, respectively, with a positive predictive value of 92% and a negative predictive value of 89%.[8]
Two or more positive initial screening tests in a patient with a high pretest probability of Cushing disease confirms the biochemical diagnosis of Cushing syndrome.[6][9] Once Cushing syndrome has been diagnosed, the first step toward finding the cause is by measuring a baseline plasma ACTH level. A level consistently greater than 3.3 pmol/L is classified as corticotropin-dependent.[8] To differentiate Cushing disease from ectopic corticotropin syndrome, a corticotropin-releasing hormone (CRH) test is needed. In a patient with Cushing disease, the administered CRH stimulates the release of additional corticotropin, resulting in an elevated plasma corticotropin level. The sensitivity of the CRH test for detecting Cushing disease is 93% when plasma levels are measured at fifteen and thirty minutes.[8] Alternatively, a high-dose 48-hour dexamethasone suppression test or pituitary magnetic resonance imaging (MRI) can be used.[8]
For high-dose 48-hour dexamethasone suppression testing, a plasma cortisol level above 50 nmol/L (measured 48-hours after either administration of dexamethasone 2 mg by mouth every 6 hours for 48 hours, or 48-hours after one dose of 8 mg is given) is indicative of Cushing disease.[2] This test has an 8% false-negative rate.[2] Pituitary MRI may show the ACTH secreting tumor if present. However, MRI fails to detect a tumor in 40% of patients with Cushing disease. The average size of the tumor that was detected on MRI was about 6 mm.[4]
The most accurate test used to differentiate a pituitary adenoma from ectopic or adrenal Cushing syndrome is inferior petrosal sinus sampling. This invasive method measures the difference in the level of ACTH found in the inferior petrosal sinus (where the pituitary gland drains) as compared to the periphery.[6][10] A basal central to the peripheral ratio of over 3:1 when CRH is administered confirms the diagnosis of Cushing disease.[10] This test is considered the gold standard in diagnosing Cushing disease because it has a sensitivity and specificity of nearly 94%, but it is rarely used in clinical practice due to its high cost, invasiveness, rare but serious complications, and required expertise to administer.[10]
If a primary ACTH secreting tumor is found, first-line treatment is surgical resection of the adenoma via trans-sphenoidal surgery (TSS).[8] This can either be conducted via an endonasal or sublabial approach, depending on surgeon preferences.[11] The probability of successful resection is higher when the tumor can be identified during the initial surgery.[11] Overall, remission rates after TSS are in the range of 65% to 90% for microadenomas and less than 65% for macroadenomas.[8] Patients with persistent disease after initial surgery frequently undergo repeat pituitary surgery despite a lower success rate and increased risk for pituitary insufficiency.[11] The most common complications of this procedure include diabetes insipidus (15%), fluid and electrolyte abnormalities (12.5%), and neurological deficits (5.6%).[11] Patients over age 64 have a higher incidence of adverse outcomes.[12]
Alternatively, pituitary radiation therapy can be used after an unsuccessful TSS.[8][13] External-beam pituitary radiotherapy is most effective in pediatric patients, with cure rates in this population as high as 80% to 88%.[14] The most common complication from this treatment is hypopituitarism, causing growth hormone deficiency. This complication has been reported in 36% to 68% of patients.[14]
Lastly, bilateral adrenalectomy can be used to provide an immediate reduction of cortisol levels in patients with Cushing disease.[2] However, these patients will then require lifelong administration of glucocorticoid and mineralocorticoid replacement therapy. A major complication of this treatment is Nelson syndrome, which is the development of ACTH secreting macroadenomas post-bilateral adrenalectomy.[2] The incidence is between 8% to 29% and is diagnosed an average of 15 years post-bilateral adrenalectomy.[2]
Post-treatment testing with 24-hour urine and blood samples are used to detect the level of cortisol.[8] The disappearance of the response to the desmopressin test after surgery may suggest complete removal of the tumor and, therefore, a lower possibility of recurrence.[15] Recurrence of hypercortisolemia occurs in about a third of patients after initial treatment of Cushing disease.[14][16] Therefore, lifelong monitoring is required. Late-night salivary cortisol appears to be the best predictor of recurrence.[17][18]
Without treatment, Cushing disease is ultimately fatal. The mortality is due to the excess production of glucocorticoids, which can lead to many medical problems, including impairment in immune function. For patients who undergo surgery, lifelong treatment with glucocorticoids is necessary.
Cushing disease is a rare pituitary gland disorder best managed by a multidisciplinary team that includes a neurosurgeon, radiation consultant, endocrinologist, radiologist, primary care provider, nurse practitioner, and an internist. These patients are prone to several complications, including peptic ulcer disease, weight gain, osteoporosis, diabetes, depressed immune system, and hypertension. Hence the patient has to be closely monitored.
Large pituitary lesions usually require resection, but small lesions may be treated with medications. These patients need lifelong follow-up with regular monitoring of cortisol levels. Recurrence of disease is not uncommon, and too much or too little cortisol can be life-threatening.[19] The pharmacist must emphasize to the patient the importance of medication compliance. The patient must also be urged to wear a Medical Alert bracelet to inform other clinicians about their health status. Patients need life long follow up. Close communication between the clinicians is vital to prevent complications and improve outcomes.
The prognosis for most patients is somewhat guarded.[20] (Level V)
Recently, medical therapy has been gaining popularity in the treatment of pituitary tumors. Although surgery is still considered the first-line treatment, pharmacological therapy can control the associated hormonal imbalances.[21] These medical therapies either target the central inhibition of ACTH secretion, adrenal inhibition of steroidogenesis, or glucocorticoid-receptor blockade. Centrally acting agents include pasireotide and cabergoline.[22] Adrenal steroidogenesis inhibitors include ketoconazole, metyrapone, etomidate, mitotane, and osilodrostat. Lastly, mifepristone can be used as a glucocorticoid-receptor blocker. Although regulatory authorities have approved several pharmaceutical treatments, their use remains limited due to high costs and associated side effects.[5]
Medical therapy has been gaining popularity in the treatment of pituitary tumors quite recently. Although surgery is still considered the first-line treatment, pharmacological therapy can control the associated hormonal imbalances.[21] These medical therapies either target the central inhibition of ACTH secretion, adrenal inhibition of steroidogenesis, or glucocorticoid-receptor blockade. Centrally acting agents include pasireotide and cabergoline.[22] Adrenal steroidogenesis inhibitors include ketoconazole, metyrapone, etomidate, mitotane, and osilodrostat. Lastly, mifepristone can be used as a glucocorticoid-receptor blocker. Although regulatory authorities have approved several pharmaceutical treatments, their use remains limited due to high costs and associated side effects.[5]
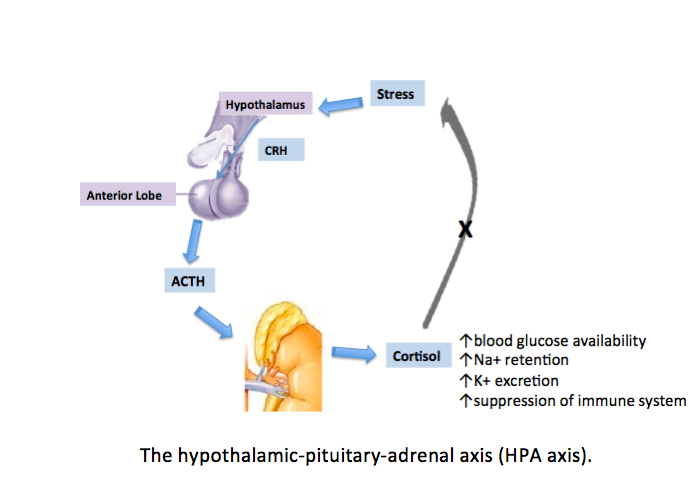
The Hypothalamic-Pituitary-Adrenal Axis
J Hine, A Schwell, N Kairys, Public Domain, via Wikimedia Commons
Hine J, Schwell A, Kairys N. An Unlikely Cause of Hypokalemia. The Journal of emergency medicine. 2017 May:52(5):e187-e191. doi: 10.1016/j.jemermed.2016.12.011. Epub 2017 Jan 28 [PubMed PMID: 28139270]
Buliman A, Tataranu LG, Paun DL, Mirica A, Dumitrache C. Cushing's disease: a multidisciplinary overview of the clinical features, diagnosis, and treatment. Journal of medicine and life. 2016 Jan-Mar:9(1):12-18 [PubMed PMID: 27974908]
Hanna FWF, Issa BG, Kevil B, Fryer AA. Investigating cortisol excess or deficiency: a practical approach. BMJ (Clinical research ed.). 2019 Nov 26:367():l6039. doi: 10.1136/bmj.l6039. Epub 2019 Nov 26 [PubMed PMID: 31771937]
Annapureddy AR, Angraal S, Caraballo C, Grimshaw A, Huang C, Mortazavi BJ, Krumholz HM. The National Institutes of Health funding for clinical research applying machine learning techniques in 2017. NPJ digital medicine. 2020:3():13. doi: 10.1038/s41746-020-0223-9. Epub 2020 Jan 31 [PubMed PMID: 32025574]
Simon J, Theodoropoulou M. Genetics of Cushing's disease. Journal of neuroendocrinology. 2022 Aug:34(8):e13148. doi: 10.1111/jne.13148. Epub 2022 May 21 [PubMed PMID: 35596671]
Schorr M, Zhang X, Zhao W, Abedi P, Lines KE, Hedley-Whyte ET, Swearingen B, Klibanski A, Miller KK, Thakker RV, Nachtigall LB. TWO SYNCHRONOUS PITUITARY ADENOMAS CAUSING CUSHING DISEASE AND ACROMEGALY. AACE clinical case reports. 2019 Sep-Oct:5(5):e276-e281. doi: 10.4158/ACCR-2019-0057. Epub 2019 Jun 7 [PubMed PMID: 31967052]
Trabzonlu L, Agirlar Trabzonlu T, Gurbuz Y, Ceylan S. ACTH-Cell Pituitary Adenoma With Signet Ring Cells: A Rare Case Report and Review of The Literature. Applied immunohistochemistry & molecular morphology : AIMM. 2020 Feb:28(2):e13-e16. doi: 10.1097/PAI.0000000000000639. Epub [PubMed PMID: 32044887]
Pappachan JM, Hariman C, Edavalath M, Waldron J, Hanna FW. Cushing's syndrome: a practical approach to diagnosis and differential diagnoses. Journal of clinical pathology. 2017 Apr:70(4):350-359. doi: 10.1136/jclinpath-2016-203933. Epub 2017 Jan 9 [PubMed PMID: 28069628]
Webb SM, Santos A, Aulinas A, Resmini E, Martel L, Martínez-Momblán MA, Valassi E. Patient-Centered Outcomes with Pituitary and Parasellar Disease. Neuroendocrinology. 2020:110(9-10):882-888. doi: 10.1159/000506809. Epub 2020 Feb 27 [PubMed PMID: 32101858]
Masopust V, Netuka D, Beneš V, Májovský M, Belšán T, Bradáč O, Hořínek D, Kosák M, Hána V, Kršek M. Magnetic resonance imaging and histology correlation in Cushing's disease. Neurologia i neurochirurgia polska. 2017 Jan-Feb:51(1):45-52. doi: 10.1016/j.pjnns.2016.10.005. Epub 2016 Nov 2 [PubMed PMID: 27988033]
Langlois F, McCartney S, Fleseriu M. Recent Progress in the Medical Therapy of Pituitary Tumors. Endocrinology and metabolism (Seoul, Korea). 2017 Jun:32(2):162-170. doi: 10.3803/EnM.2017.32.2.162. Epub [PubMed PMID: 28685507]
Wagner-Bartak NA, Baiomy A, Habra MA, Mukhi SV, Morani AC, Korivi BR, Waguespack SG, Elsayes KM. Cushing Syndrome: Diagnostic Workup and Imaging Features, With Clinical and Pathologic Correlation. AJR. American journal of roentgenology. 2017 Jul:209(1):19-32. doi: 10.2214/AJR.16.17290. Epub [PubMed PMID: 28639924]
Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR, Boguszewski CL, Bronstein MD, Buchfelder M, Carmichael JD, Casanueva FF, Castinetti F, Chanson P, Findling J, Gadelha M, Geer EB, Giustina A, Grossman A, Gurnell M, Ho K, Ioachimescu AG, Kaiser UB, Karavitaki N, Katznelson L, Kelly DF, Lacroix A, McCormack A, Melmed S, Molitch M, Mortini P, Newell-Price J, Nieman L, Pereira AM, Petersenn S, Pivonello R, Raff H, Reincke M, Salvatori R, Scaroni C, Shimon I, Stratakis CA, Swearingen B, Tabarin A, Takahashi Y, Theodoropoulou M, Tsagarakis S, Valassi E, Varlamov EV, Vila G, Wass J, Webb SM, Zatelli MC, Biller BMK. Consensus on diagnosis and management of Cushing's disease: a guideline update. The lancet. Diabetes & endocrinology. 2021 Dec:9(12):847-875. doi: 10.1016/S2213-8587(21)00235-7. Epub 2021 Oct 20 [PubMed PMID: 34687601]
Lu L, Chen JH, Zhu HJ, Song AL, Li M, Chen S, Pan H, Gong FY, Wang RZ, Xing B, Yao Y, Feng M, Lu ZL. [Comparison of efficacy between the serum cortisol and 24 hour urine free cortisol in combined dexamethasone suppression test in the diagnosis of Cushing syndrome]. Zhonghua yi xue za zhi. 2016 Jul 19:96(27):2150-4. doi: 10.3760/cma.j.issn.0376-2491.2016.27.008. Epub [PubMed PMID: 27464539]
Molitch ME. Diagnosis and Treatment of Pituitary Adenomas: A Review. JAMA. 2017 Feb 7:317(5):516-524. doi: 10.1001/jama.2016.19699. Epub [PubMed PMID: 28170483]
Braun LT, Riester A, Oßwald-Kopp A, Fazel J, Rubinstein G, Bidlingmaier M, Beuschlein F, Reincke M. Toward a Diagnostic Score in Cushing's Syndrome. Frontiers in endocrinology. 2019:10():766. doi: 10.3389/fendo.2019.00766. Epub 2019 Nov 8 [PubMed PMID: 31787931]
Vassiliadi DA, Balomenaki M, Asimakopoulou A, Botoula E, Tzanela M, Tsagarakis S. The Desmopressin Test Predicts Better Than Basal Cortisol the Long-Term Surgical Outcome of Cushing's Disease. The Journal of clinical endocrinology and metabolism. 2016 Dec:101(12):4878-4885 [PubMed PMID: 27662440]
Lad SP, Patil CG, Laws ER Jr, Katznelson L. The role of inferior petrosal sinus sampling in the diagnostic localization of Cushing's disease. Neurosurgical focus. 2007:23(3):E2 [PubMed PMID: 17961020]
Losa M, Albano L, Bailo M, Barzaghi LR, Mortini P. Role of radiosurgery in the treatment of Cushing's disease. Journal of neuroendocrinology. 2022 Aug:34(8):e13134. doi: 10.1111/jne.13134. Epub 2022 Aug 18 [PubMed PMID: 35980263]
Ye VC, Akagami R. Perioperative Quality of Life in Cushing's Disease. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2017 Jan:44(1):69-77. doi: 10.1017/cjn.2016.295. Epub 2016 Sep 20 [PubMed PMID: 27645104]
Guignat L, Bertherat J. Long-term follow-up and predictors of recurrence of Cushing's disease. Journal of neuroendocrinology. 2022 Aug:34(8):e13186. doi: 10.1111/jne.13186. Epub 2022 Aug 18 [PubMed PMID: 35979714]
Ciato D, Mumbach AG, Paez-Pereda M, Stalla GK. Currently used and investigational drugs for Cushing´s disease. Expert opinion on investigational drugs. 2017 Jan:26(1):75-84. doi: 10.1080/13543784.2017.1266338. Epub 2016 Dec 8 [PubMed PMID: 27894193]
Minniti G, Osti MF, Niyazi M. Target delineation and optimal radiosurgical dose for pituitary tumors. Radiation oncology (London, England). 2016 Oct 11:11(1):135 [PubMed PMID: 27729088]
Yordanova G, Martin L, Afshar F, Sabin I, Alusi G, Plowman NP, Riddoch F, Evanson J, Matson M, Grossman AB, Akker SA, Monson JP, Drake WM, Savage MO, Storr HL. Long-term outcomes of children treated for Cushing's disease: a single center experience. Pituitary. 2016 Dec:19(6):612-624 [PubMed PMID: 27678103]
Honegger J, Nasi-Kordhishti I. Surgery and perioperative management of patients with Cushing's disease. Journal of neuroendocrinology. 2022 Aug:34(8):e13177. doi: 10.1111/jne.13177. Epub 2022 Aug 18 [PubMed PMID: 35980172]
Abellán Galiana P, Fajardo Montañana C, Riesgo Suárez PA, Gómez Vela J, Escrivá CM, Lillo VR. [Predictors of long-term remission after transsphenoidal surgery in Cushing's disease]. Endocrinologia y nutricion : organo de la Sociedad Espanola de Endocrinologia y Nutricion. 2013 Oct:60(8):475-82. doi: 10.1016/j.endonu.2012.09.009. Epub 2012 Dec 23 [PubMed PMID: 23266144]
Kuo CH, Shih SR, Li HY, Chen SC, Hung PJ, Tseng FY, Chang TC. Adrenocorticotropic hormone levels before treatment predict recurrence of Cushing's disease. Journal of the Formosan Medical Association = Taiwan yi zhi. 2017 Jun:116(6):441-447. doi: 10.1016/j.jfma.2016.08.008. Epub 2016 Oct 28 [PubMed PMID: 28029519]
Bertherat J. THE CHALLENGE OF EARLY DIAGNOSIS OF CUSHING DISEASE RECURRENCE! Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2016 Nov:22(11):1356-1357 [PubMed PMID: 27749130]
Fleseriu M, Hamrahian AH, Hoffman AR, Kelly DF, Katznelson L, AACE Neuroendocrine and Pituitary Scientific Committee *. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY DISEASE STATE CLINICAL REVIEW: DIAGNOSIS OF RECURRENCE IN CUSHING DISEASE. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2016 Dec:22(12):1436-1448 [PubMed PMID: 27643842]
Grabner P, Hauer-Jensen M, Jervell J, Flatmark A. Long-term results of treatment of Cushing's disease by adrenalectomy. The European journal of surgery = Acta chirurgica. 1991 Aug:157(8):461-4 [PubMed PMID: 1681932]
Ferriere A, Cortet C, Chanson P, Delemer B, Caron P, Chabre O, Reznik Y, Bertherat J, Rohmer V, Briet C, Raingeard I, Castinetti F, Beckers A, Vroonen L, Maiter D, Cephise-Velayoudom FL, Nunes ML, Haissaguerre M, Tabarin A. Cabergoline for Cushing's disease: a large retrospective multicenter study. European journal of endocrinology. 2017 Mar:176(3):305-314. doi: 10.1530/EJE-16-0662. Epub 2016 Dec 22 [PubMed PMID: 28007845]
von Selzam V, Theodoropoulou M. Innovative tumour targeting therapeutics in Cushing's disease. Best practice & research. Clinical endocrinology & metabolism. 2022 Dec:36(6):101701. doi: 10.1016/j.beem.2022.101701. Epub 2022 Sep 9 [PubMed PMID: 36511278]
Lacroix A, Gu F, Gallardo W, Pivonello R, Yu Y, Witek P, Boscaro M, Salvatori R, Yamada M, Tauchmanova L, Roughton M, Ravichandran S, Petersenn S, Biller BMK, Newell-Price J, Pasireotide G2304 Study Group. Efficacy and safety of once-monthly pasireotide in Cushing's disease: a 12 month clinical trial. The lancet. Diabetes & endocrinology. 2018 Jan:6(1):17-26. doi: 10.1016/S2213-8587(17)30326-1. Epub 2017 Oct 12 [PubMed PMID: 29032078]
Lacroix A, Bronstein MD, Schopohl J, Delibasi T, Salvatori R, Li Y, Barkan A, Suzaki N, Tauchmanova L, Ortmann CE, Ravichandran S, Petersenn S, Pivonello R. Long-acting pasireotide improves clinical signs and quality of life in Cushing's disease: results from a phase III study. Journal of endocrinological investigation. 2020 Nov:43(11):1613-1622. doi: 10.1007/s40618-020-01246-0. Epub 2020 May 8 [PubMed PMID: 32385851]
Hinojosa-Amaya JM, Johnson N, González-Torres C, Varlamov EV, Yedinak CG, McCartney S, Fleseriu M. Depression and Impulsivity Self-Assessment Tools to Identify Dopamine Agonist Side Effects in Patients With Pituitary Adenomas. Frontiers in endocrinology. 2020:11():579606. doi: 10.3389/fendo.2020.579606. Epub 2020 Oct 27 [PubMed PMID: 33193096]
Castinetti F, Guignat L, Giraud P, Muller M, Kamenicky P, Drui D, Caron P, Luca F, Donadille B, Vantyghem MC, Bihan H, Delemer B, Raverot G, Motte E, Philippon M, Morange I, Conte-Devolx B, Quinquis L, Martinie M, Vezzosi D, Le Bras M, Baudry C, Christin-Maitre S, Goichot B, Chanson P, Young J, Chabre O, Tabarin A, Bertherat J, Brue T. Ketoconazole in Cushing's disease: is it worth a try? The Journal of clinical endocrinology and metabolism. 2014 May:99(5):1623-30. doi: 10.1210/jc.2013-3628. Epub 2014 Jan 28 [PubMed PMID: 24471573]
Young J, Bertherat J, Vantyghem MC, Chabre O, Senoussi S, Chadarevian R, Castinetti F, Compassionalte use Programme. Hepatic safety of ketoconazole in Cushing's syndrome: results of a Compassionate Use Programme in France. European journal of endocrinology. 2018 May:178(5):447-458. doi: 10.1530/EJE-17-0886. Epub 2018 Feb 22 [PubMed PMID: 29472378]
Rasool S, Skinner BW. Osilodrostat for the treatment of Cushing's disease. Expert opinion on pharmacotherapy. 2021 Jun:22(9):1099-1106. doi: 10.1080/14656566.2021.1897106. Epub 2021 Mar 11 [PubMed PMID: 33703978]
Pivonello R, Fleseriu M, Newell-Price J, Bertagna X, Findling J, Shimatsu A, Gu F, Auchus R, Leelawattana R, Lee EJ, Kim JH, Lacroix A, Laplanche A, O'Connell P, Tauchmanova L, Pedroncelli AM, Biller BMK, LINC 3 investigators. Efficacy and safety of osilodrostat in patients with Cushing's disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. The lancet. Diabetes & endocrinology. 2020 Sep:8(9):748-761. doi: 10.1016/S2213-8587(20)30240-0. Epub 2020 Jul 27 [PubMed PMID: 32730798]
Fleseriu M, Findling JW, Koch CA, Schlaffer SM, Buchfelder M, Gross C. Changes in plasma ACTH levels and corticotroph tumor size in patients with Cushing's disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone. The Journal of clinical endocrinology and metabolism. 2014 Oct:99(10):3718-27. doi: 10.1210/jc.2014-1843. Epub 2014 Jul 11 [PubMed PMID: 25013998]
Guarda FJ, Findling J, Yuen KCJ, Fleseriu M, Nachtigall LB. Mifepristone Increases Thyroid Hormone Requirements in Patients With Central Hypothyroidism: A Multicenter Study. Journal of the Endocrine Society. 2019 Sep 1:3(9):1707-1714. doi: 10.1210/js.2019-00188. Epub 2019 Jul 5 [PubMed PMID: 31528830]
Coulden A, Hamblin R, Wass J, Karavitaki N. Cardiovascular health and mortality in Cushing's disease. Pituitary. 2022 Oct:25(5):750-753. doi: 10.1007/s11102-022-01258-4. Epub 2022 Jul 22 [PubMed PMID: 35869339]
Tritos NA, Biller BMK. Medical Management of Cushing Disease. Neurosurgery clinics of North America. 2019 Oct:30(4):499-508. doi: 10.1016/j.nec.2019.05.007. Epub 2019 Jul 5 [PubMed PMID: 31471057]
Vega-Beyhart A, Enriquez-Estrada VM, Bello-Chavolla OY, Torres-Victoria TR, Martínez-Sánchez FD, López-Navarro JM, Pérez-Guzmán MC, Hinojosa-Amaya JM, León-Suárez A, Espinoza-Salazar HD, Roldán-Sarmiento P, Gómez-Sámano MA, Gómez-Pérez FJ, Cuevas-Ramos D. Quality of life is significantly impaired in both secretory and non-functioning pituitary adenomas. Clinical endocrinology. 2019 Mar:90(3):457-467. doi: 10.1111/cen.13915. Epub 2019 Jan 15 [PubMed PMID: 30548674]
Rotman LE, Vaughan TB, Hackney JR, Riley KO. Long-Term Survival After Transformation of an Adrenocorticotropic Hormone-Secreting Pituitary Macroadenoma to a Silent Corticotroph Pituitary Carcinoma. World neurosurgery. 2019 Feb:122():417-423. doi: 10.1016/j.wneu.2018.11.011. Epub 2018 Nov 14 [PubMed PMID: 30447452]