Learning Outcome
- Describe the symptoms of AD
- Recall the management of a patient with AD
- Describe the cognitive issues in AD
- Summarize the role of nurses in managing AD

Dementia is a general term that refers to a decline in cognitive ability severe enough to interfere with activities of daily living. Alzheimer disease (AD) is the most common type of dementia, accounting for at least two-thirds of cases of dementia in people age 65 and older. Alzheimer disease is a neurodegenerative disease that causes progressive and disabling impairment of cognitive functions including memory, comprehension, language, attention, reasoning, and judgment. It is the sixth leading cause of death in the United States. Alzheimer disease is typically a disease of old age. Onset before 65 years of age (early onset) is unusual and seen in less than 10% of Alzheimer disease patients. The most common presenting symptom is selective short-term memory loss. The disease is invariably progressive, eventually leading to severe cognitive decline. There is no cure for Alzheimer disease, although there are treatments available that may improve some symptoms.
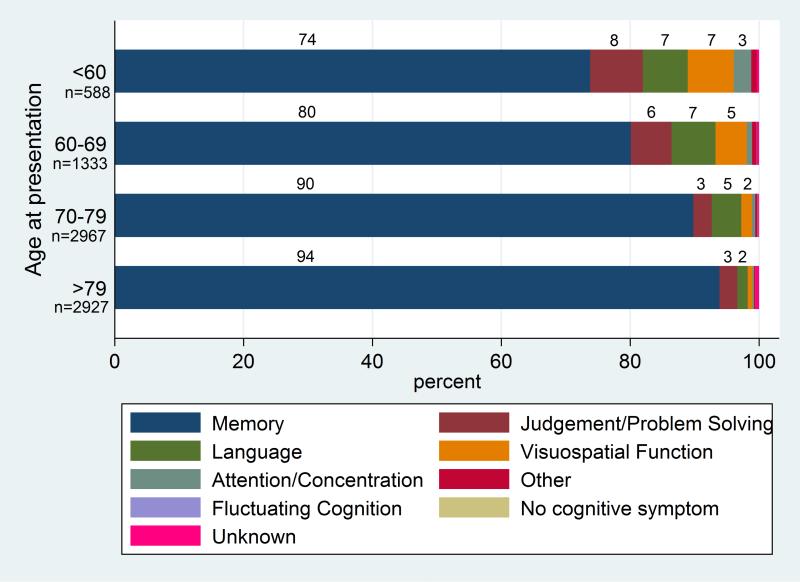
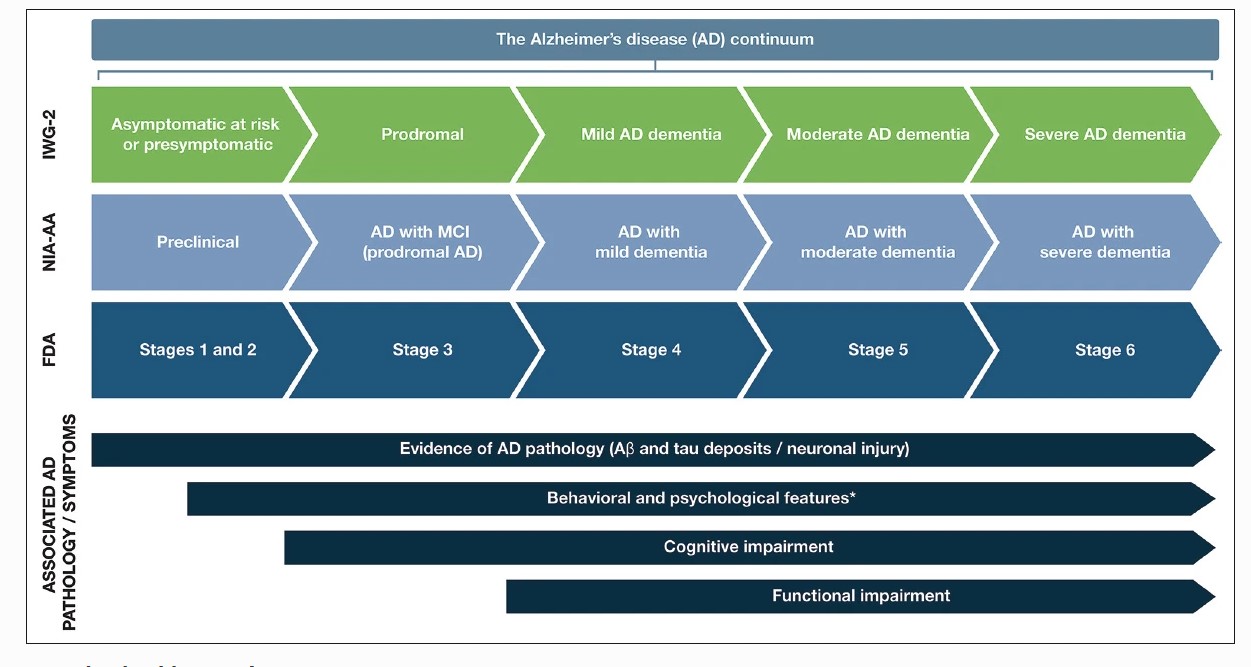
Symptoms of Alzheimer disease depend on the stage of the disease. Alzheimer disease is classified into preclinical, mild, moderate, and late-stage depending on the degree of cognitive impairment. The initial presenting symptom is usually recent memory loss with relative sparing of long-term memory and can be elicited in most patients even when not the presenting symptom. Short-term memory impairment is followed by impairment in problem-solving, judgment, executive functioning, lack of motivation and disorganization, leading to problems with multitasking and abstract thinking. In the early stages, impairment in executive functioning may be subtle. This is followed by language disorder and impairment of visuospatial skills. Neuropsychiatric symptoms like apathy, social withdrawal, disinhibition, agitation, psychosis, and wandering are also common in mid to late stages. Difficulty performing learned motor tasks (dyspraxia), olfactory dysfunction, sleep disturbances, extrapyramidal motor signs like dystonia, akathisia, and parkinsonian symptoms occur late in the disease. This is followed by primitive reflexes, incontinence, and total dependence on caregivers.[1],[2],[3]
Alzheimer disease is a gradual and progressive neurodegenerative disease caused by neuronal cell death. It typically starts in the entorhinal cortex in the hippocampus. There is a genetic role identified for both early and late-onset Alzheimer disease. Several risk factors have been associated with Alzheimer disease. Increasing age is the most important risk factor for Alzheimer disease. Traumatic head injury, depression, cardiovascular and cerebrovascular disease, higher parental age, smoking, family history of dementia, and presence of APOE e4 allele are known to increase the risk of Alzheimer disease. Higher education, use of estrogen by women, use of anti-inflammatory agents, and regular aerobic exercise is known to decrease the risk of Alzheimer disease. Having a first-degree relative with Alzheimer disease increases the risk of developing Alzheimer disease by 10% to 30%. Individuals with 2 or more siblings with late-onset Alzheimer disease increases their risk of getting Alzheimer disease by 3-fold as compared to the general population.[4],[5],[6]
Alzheimer disease is typically a disease of old age. The global prevalence of dementia is reported to be as high as 24 million and is predicted to increase 4 times by the year 2050. Estimated health care cost of Alzheimer disease is $172 billion per year in the United States alone. In 2011, the United States had an estimated 4.5 million people age sixty-five and above, living with clinical Alzheimer disease. The incidence of dementia is predicted to double every 10 years after 60 years of age. Age-specific incidence increases significantly from less than 1% per year before 65 years of age to 6% per year after 85 years of age. Incidence rates of Alzheimer disease are slightly higher for women, especially after 85 years of age.
A good history and physical examination are the keys to diagnosis. It is also essential to take a history from the family and caregivers as some patients may lack insight into their disease. It is vital to characterize onset and early symptoms to differentiate from other types of dementia. It is important to obtain a good assessment of functional abilities like basic and individual activities of daily living.
A complete physical examination with a detailed neurological exam and mental status examination is needed to evaluate disease stage and rule out other conditions. Comprehensive clinical assessment can provide reasonable diagnostic accuracy in most patients. A detailed neurological examination is essential to rule out other conditions. In Alzheimer disease, the neurological exam is usually normal. A mental status examination should assess concentration, attention, recent and remote memory, language, visuospatial functioning, praxis, and executive functioning.
Brief standard examinations like the mini-mental status examination are less sensitive and specific, although they can be used for screening.
All follow-up visits should include a full mental status examination to evaluate disease progression and development of neuropsychiatric symptoms.
Routine laboratory tests show no specific abnormality. Complete blood count (CBC), complete metabolic panel (CMP), thyroid-stimulating hormone (TSH), B12 are usually checked to rule out other causes.[7],[8],[9]
Brain imaging may help in the diagnosis and monitor clinical course of the disease. MRI or CT brain can help exclude other causes of dementia like stroke or tumors. Dilated lateral ventricles and widened cortical sulci, especially in the temporal area are typical for Alzheimer disease.
Cerebrospinal fluid (CSF) is usually normal, but total protein may be mildly elevated. Measurements of total-tau, beta-amyloid, and phosphorylated tau protein are sometimes helpful for differential diagnosis. Alzheimer disease is strongly predicted if CSF has decreased beta-amyloid 42 and increased tau protein.
EEG typically shows a generalized slowing with no focal features.
Most reliable method to detect mild cognitive impairment in early disease is neuropsychological testing.
More recently, volumetric MRI is being used to precisely measure volumetric changes in the brain. In Alzheimer disease, volumetric MRI shows shrinkage in the medial temporal lobe. However, hippocampal atrophy is also linked to normal age-related memory decline, so the use of volumetric MRI for early detection of Alzheimer disease is questionable. A definite role for volumetric MRI to aid diagnosis of Alzheimer disease is not fully established yet.
Functional brain imaging techniques like PET, fMRI, and SPECT are being used to map patterns of dysfunction in smaller brain areas of the medial temporal lobe. These studies may be helpful in early detection and monitoring clinical course; however, their role in the diagnosis of Alzheimer disease is not fully established yet.
Most recently, there have been developments in brain imaging techniques to detect core histological features of Alzheimer disease, that is amyloid plaques and neurofibrillary tangles. The utility of these techniques is still being investigated.
Genetic testing is usually not recommended for Alzheimer disease. It may sometimes be used in families with rare early-onset forms of Alzheimer disease.
It is important to understand that diagnosing the type of dementia with all certainty may not be entirely possible despite excellent clinical history, physical examination and relevant testing. Some patients will complain of cognitive impairment that can be verified objectively, but is not severe enough to impair activities of daily life and thus does not meet criteria for dementia, and is usually just classified as mild cognitive impairment. However, a significant proportion of people with mild cognitive impairment will develop dementia of some type in 5 to 7 years.
There is no cure for Alzheimer disease. Only symptomatic treatment is available.[10][11][12]
Two categories of drugs are approved for treatment of Alzheimer disease: cholinesterase inhibitors and partial N-methyl D-aspartate (NMDA) antagonists.
Cholinesterase Inhibitors
Cholinesterase inhibitors act by increasing the level of acetylcholine; a chemical used by nerve cells to communicate with each other and is important for learning, memory and cognitive functions. Of this category, 3 drugs: donepezil, rivastigmine, and galantamine are FDA-approved for treatment of Alzheimer disease.
Donepezil can be used in all stages of Alzheimer disease. Galantamine and rivastigmine are approved for treatment in mild to moderate Alzheimer disease only. Donepezil and galantamine are rapid, reversible inhibitors of acetylcholinesterase. Rivastigmine is a slow, reversible inhibitor of acetylcholinesterase and butyrylcholinesterase. Donepezil is usually preferred of all because of once-daily dosing. Galantamine is available as a twice daily tablet or as a once-daily extended-release capsule. It cannot be used in end-stage renal disease or severe liver dysfunction. Rivastigmine is available in an oral and transdermal formulation. Most common side effects of cholinesterase inhibitors are gastrointestinal-like nausea, vomiting, and diarrhea. Sleep disturbances are more common with donepezil. Due to increased vagal tone, bradycardia, cardiac conduction defects, and syncope can occur, and these medications are contraindicated in patients with severe cardiac conduction abnormalities.
Partial N-Methyl D-Aspartate (NMDA) Memantine
Partial N-Methyl D-aspartate (NMDA) antagonist memantine blocks NMDA receptors and slows intracellular calcium accumulation. It is approved by FDA for treating moderate to severe Alzheimer disease. Dizziness, body aches, headache, and constipation are common side effects. It can be taken in combination with cholinesterase inhibitors.[13]
It is also important to treat anxiety, depression, and psychosis, which is often found in mid to late stages of Alzheimer disease.
Environmental and behavioral approaches are beneficial especially in managing behavioral problems. Simple approaches such as maintaining a familiar environment, monitoring personal comfort, providing security object, redirecting attention, and avoiding confrontation can be very helpful in managing behavioral issues.
The expected benefits of treatment are modest. Treatment should be stopped or modified if no significant benefits or if intolerable side effects.
Regular aerobic exercise has been shown to slow the progression of Alzheimer disease.
Alzheimer disease (AD) is a progressive neurodegenerative disorder marked by behavior and cognitive impairment that eventually interfere with daily functional living activities. The disorder has no cure, and its rate of progression is variable. Further, the diagnosis of Alzheimer disease in the early phase is difficult, and there are no specific laboratory or imaging tests to confirm the diagnosis. The drugs available to treat the condition only work for the mild disease but also have numerous side effects which are not well tolerated. Alzheimer disease is a systemic disorder and creates havoc in the family. These individuals often wander, fall, have significant behavior problems and loss of memory. The majority of patients end up in an institution because they become unmanageable at home. Because of the nature of the disease, an interprofessional approach to the disorder has been recommended. Many guidelines and recommendations have been made on how to approach, monitor and treat Alzheimer patients. No one measure can prevent or arrest the disease. Given this, the following health care workers have a critical role in ensuring that the patient with Alzheimer disease remains safe and lead a decent quality of life.
Physical therapy for exercise. There is now ample evidence that exercise can help reduce the progression of the disease.[14] (Level III)
Nurses to educate the patient and family on medications, lifestyle changes, and performing daily living activities. To educate the partner on self-reporting on the worsening of symptoms.
Pharmacist to ensure that polypharmacy does not occur and that the patient is not developing adverse effects.
Outcomes
Alzheimer disease is initially associated only with impaired memory, but with time, the individual may develop severe cognitive and behavioral symptoms like depression, anxiety, anger, irritability, insomnia, and paranoia. As the disease progresses most of them will require assistance with daily living activities. Eventually, even walking become difficult and many may not be able to eat or develop swallowing difficulties that lead to aspiration pneumonia.
The time from diagnosis to death is variable; some individuals may die within five years, and others may remain alive for ten years, but overall the quality of life is very poor. While an interprofessional approach to management of Alzheimer patients is recommended, an analysis of several studies reveals that this approach has no impact on the care of his patients. However, because of the heterogeneity in the previous studies, more robust studies will be required to determine what type of approach works best for managing these patients.[15]
Educate the family and caregiver about the support systems
Ahmad FB, Cisewski JA, Xu J, Anderson RN. Provisional Mortality Data - United States, 2022. MMWR. Morbidity and mortality weekly report. 2023 May 5:72(18):488-492. doi: 10.15585/mmwr.mm7218a3. Epub 2023 May 5 [PubMed PMID: 37141156]
Mendez MF. Early-Onset Alzheimer Disease. Neurologic clinics. 2017 May:35(2):263-281. doi: 10.1016/j.ncl.2017.01.005. Epub [PubMed PMID: 28410659]
Zetterberg H, Bendlin BB. Biomarkers for Alzheimer's disease-preparing for a new era of disease-modifying therapies. Molecular psychiatry. 2021 Jan:26(1):296-308. doi: 10.1038/s41380-020-0721-9. Epub 2020 Apr 6 [PubMed PMID: 32251378]
Porsteinsson AP, Isaacson RS, Knox S, Sabbagh MN, Rubino I. Diagnosis of Early Alzheimer's Disease: Clinical Practice in 2021. The journal of prevention of Alzheimer's disease. 2021:8(3):371-386. doi: 10.14283/jpad.2021.23. Epub [PubMed PMID: 34101796]
Therriault J, Zimmer ER, Benedet AL, Pascoal TA, Gauthier S, Rosa-Neto P. Staging of Alzheimer's disease: past, present, and future perspectives. Trends in molecular medicine. 2022 Sep:28(9):726-741. doi: 10.1016/j.molmed.2022.05.008. Epub 2022 Jun 15 [PubMed PMID: 35717526]
Tang Y, Lutz MW, Xing Y. A systems-based model of Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2019 Jan:15(1):168-171. doi: 10.1016/j.jalz.2018.06.3058. Epub 2018 Aug 10 [PubMed PMID: 30102884]
Zilberzwige-Tal S, Gazit E. Go with the Flow-Microfluidics Approaches for Amyloid Research. Chemistry, an Asian journal. 2018 Nov 16:13(22):3437-3447. doi: 10.1002/asia.201801007. Epub 2018 Sep 24 [PubMed PMID: 30117682]
Maccioni RB, González A, Andrade V, Cortés N, Tapia JP, Guzmán-Martínez L. Alzheimer´s Disease in the Perspective of Neuroimmunology. The open neurology journal. 2018:12():50-56. doi: 10.2174/1874205X01812010050. Epub 2018 Jun 29 [PubMed PMID: 30069256]
Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer's disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimer's & dementia (Amsterdam, Netherlands). 2017:7():69-87. doi: 10.1016/j.dadm.2017.01.005. Epub 2017 Feb 9 [PubMed PMID: 28275702]
Anjum I, Fayyaz M, Wajid A, Sohail W, Ali A. Does Obesity Increase the Risk of Dementia: A Literature Review. Cureus. 2018 May 21:10(5):e2660. doi: 10.7759/cureus.2660. Epub 2018 May 21 [PubMed PMID: 30042911]
Nicolas G, Acuña-Hidalgo R, Keogh MJ, Quenez O, Steehouwer M, Lelieveld S, Rousseau S, Richard AC, Oud MS, Marguet F, Laquerrière A, Morris CM, Attems J, Smith C, Ansorge O, Al Sarraj S, Frebourg T, Campion D, Hannequin D, Wallon D, Gilissen C, Chinnery PF, Veltman JA, Hoischen A. Somatic variants in autosomal dominant genes are a rare cause of sporadic Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2018 Dec:14(12):1632-1639. doi: 10.1016/j.jalz.2018.06.3056. Epub 2018 Aug 13 [PubMed PMID: 30114415]
Liljegren M, Landqvist Waldö M, Rydbeck R, Englund E. Police Interactions Among Neuropathologically Confirmed Dementia Patients: Prevalence and Cause. Alzheimer disease and associated disorders. 2018 Oct-Dec:32(4):346-350. doi: 10.1097/WAD.0000000000000267. Epub [PubMed PMID: 30095442]
Tong BC, Wu AJ, Li M, Cheung KH. Calcium signaling in Alzheimer's disease & therapies. Biochimica et biophysica acta. Molecular cell research. 2018 Nov:1865(11 Pt B):1745-1760. doi: 10.1016/j.bbamcr.2018.07.018. Epub 2018 Jul 29 [PubMed PMID: 30059692]
Li X, Feng X, Sun X, Hou N, Han F, Liu Y. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990-2019. Frontiers in aging neuroscience. 2022:14():937486. doi: 10.3389/fnagi.2022.937486. Epub 2022 Oct 10 [PubMed PMID: 36299608]
Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues in clinical neuroscience. 2009:11(2):111-28 [PubMed PMID: 19585947]
Breijyeh Z, Karaman R. Comprehensive Review on Alzheimer's Disease: Causes and Treatment. Molecules (Basel, Switzerland). 2020 Dec 8:25(24):. doi: 10.3390/molecules25245789. Epub 2020 Dec 8 [PubMed PMID: 33302541]
Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ, Khachaturian ZS. The cholinergic system in the pathophysiology and treatment of Alzheimer's disease. Brain : a journal of neurology. 2018 Jul 1:141(7):1917-1933. doi: 10.1093/brain/awy132. Epub [PubMed PMID: 29850777]
Paroni G, Bisceglia P, Seripa D. Understanding the Amyloid Hypothesis in Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2019:68(2):493-510. doi: 10.3233/JAD-180802. Epub [PubMed PMID: 30883346]
Salehi A, Ashford JW, Mufson EJ. The Link between Alzheimer's Disease and Down Syndrome. A Historical Perspective. Current Alzheimer research. 2016:13(1):2-6 [PubMed PMID: 26487155]
Hoogmartens J, Cacace R, Van Broeckhoven C. Insight into the genetic etiology of Alzheimer's disease: A comprehensive review of the role of rare variants. Alzheimer's & dementia (Amsterdam, Netherlands). 2021:13(1):e12155. doi: 10.1002/dad2.12155. Epub 2021 Feb 20 [PubMed PMID: 33665345]
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2011 Sep:1(1):a006189. doi: 10.1101/cshperspect.a006189. Epub [PubMed PMID: 22229116]
Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. Journal of neuropathology and experimental neurology. 2011 Nov:70(11):960-9. doi: 10.1097/NEN.0b013e318232a379. Epub [PubMed PMID: 22002422]
Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA neurology. 2014 Apr:71(4):505-8. doi: 10.1001/jamaneurol.2013.5847. Epub [PubMed PMID: 24493463]
Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nature reviews. Neurology. 2020 Jan:16(1):30-42. doi: 10.1038/s41582-019-0281-2. Epub 2019 Dec 11 [PubMed PMID: 31827267]
Verma M, Wills Z, Chu CT. Excitatory Dendritic Mitochondrial Calcium Toxicity: Implications for Parkinson's and Other Neurodegenerative Diseases. Frontiers in neuroscience. 2018:12():523. doi: 10.3389/fnins.2018.00523. Epub 2018 Aug 2 [PubMed PMID: 30116173]
Wallace L, Theou O, Rockwood K, Andrew MK. Relationship between frailty and Alzheimer's disease biomarkers: A scoping review. Alzheimer's & dementia (Amsterdam, Netherlands). 2018:10():394-401. doi: 10.1016/j.dadm.2018.05.002. Epub 2018 May 30 [PubMed PMID: 30094326]
Vik-Mo AO, Bencze J, Ballard C, Hortobágyi T, Aarsland D. Advanced cerebral amyloid angiopathy and small vessel disease are associated with psychosis in Alzheimer's disease. Journal of neurology, neurosurgery, and psychiatry. 2019 Jun:90(6):728-730. doi: 10.1136/jnnp-2018-318445. Epub 2018 Jul 27 [PubMed PMID: 30054314]
Santacruz KS, Swagerty D. Early diagnosis of dementia. American family physician. 2001 Feb 15:63(4):703-13, 717-8 [PubMed PMID: 11237085]
Jia X, Wang Z, Huang F, Su C, Du W, Jiang H, Wang H, Wang J, Wang F, Su W, Xiao H, Wang Y, Zhang B. A comparison of the Mini-Mental State Examination (MMSE) with the Montreal Cognitive Assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: a cross-sectional study. BMC psychiatry. 2021 Oct 4:21(1):485. doi: 10.1186/s12888-021-03495-6. Epub 2021 Oct 4 [PubMed PMID: 34607584]
Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kędziora-Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatria polska. 2016 Oct 31:50(5):1039-1052. doi: 10.12740/PP/45368. Epub [PubMed PMID: 27992895]
Limpawattana P, Manjavong M. The Mini-Cog, Clock Drawing Test, and Three-Item Recall Test: Rapid Cognitive Screening Tools with Comparable Performance in Detecting Mild NCD in Older Patients. Geriatrics (Basel, Switzerland). 2021 Sep 16:6(3):. doi: 10.3390/geriatrics6030091. Epub 2021 Sep 16 [PubMed PMID: 34562992]
Haapasalo A, Hiltunen M. A report from the 8th Kuopio Alzheimer Symposium. Neurodegenerative disease management. 2018 Oct:8(5):289-299. doi: 10.2217/nmt-2018-0029. Epub 2018 Aug 16 [PubMed PMID: 30112972]
Kim H. Detection of severity in Alzheimer's disease (AD) using computational modeling. Bioinformation. 2018:14(5):259-264. doi: 10.6026/97320630014259. Epub 2018 May 31 [PubMed PMID: 30108425]
Petersen RC. How early can we diagnose Alzheimer disease (and is it sufficient)? The 2017 Wartenberg lecture. Neurology. 2018 Aug 28:91(9):395-402. doi: 10.1212/WNL.0000000000006088. Epub 2018 Aug 8 [PubMed PMID: 30089620]
Sobański M, Zacharzewska-Gondek A, Waliszewska-Prosół M, Sąsiadek MJ, Zimny A, Bladowska J. A Review of Neuroimaging in Rare Neurodegenerative Diseases. Dementia and geriatric cognitive disorders. 2020:49(6):544-556. doi: 10.1159/000512543. Epub 2021 Jan 28 [PubMed PMID: 33508841]
Bottino CM, Castro CC, Gomes RL, Buchpiguel CA, Marchetti RL, Neto MR. Volumetric MRI measurements can differentiate Alzheimer's disease, mild cognitive impairment, and normal aging. International psychogeriatrics. 2002 Mar:14(1):59-72 [PubMed PMID: 12094908]
Frisoni GB, Boccardi M, Barkhof F, Blennow K, Cappa S, Chiotis K, Démonet JF, Garibotto V, Giannakopoulos P, Gietl A, Hansson O, Herholz K, Jack CR Jr, Nobili F, Nordberg A, Snyder HM, Ten Kate M, Varrone A, Albanese E, Becker S, Bossuyt P, Carrillo MC, Cerami C, Dubois B, Gallo V, Giacobini E, Gold G, Hurst S, Lönneborg A, Lovblad KO, Mattsson N, Molinuevo JL, Monsch AU, Mosimann U, Padovani A, Picco A, Porteri C, Ratib O, Saint-Aubert L, Scerri C, Scheltens P, Schott JM, Sonni I, Teipel S, Vineis P, Visser PJ, Yasui Y, Winblad B. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. The Lancet. Neurology. 2017 Aug:16(8):661-676. doi: 10.1016/S1474-4422(17)30159-X. Epub 2017 Jul 11 [PubMed PMID: 28721928]
Johnson KA, Fox NC, Sperling RA, Klunk WE. Brain imaging in Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2012 Apr:2(4):a006213. doi: 10.1101/cshperspect.a006213. Epub [PubMed PMID: 22474610]
Blennow K, Zetterberg H. Biomarkers for Alzheimer's disease: current status and prospects for the future. Journal of internal medicine. 2018 Dec:284(6):643-663. doi: 10.1111/joim.12816. Epub 2018 Aug 19 [PubMed PMID: 30051512]
Hussein W, Sağlık BN, Levent S, Korkut B, Ilgın S, Özkay Y, Kaplancıklı ZA. Synthesis and Biological Evaluation of New Cholinesterase Inhibitors for Alzheimer's Disease. Molecules (Basel, Switzerland). 2018 Aug 14:23(8):. doi: 10.3390/molecules23082033. Epub 2018 Aug 14 [PubMed PMID: 30110946]
Leblhuber F, Steiner K, Schuetz B, Fuchs D, Gostner JM. Probiotic Supplementation in Patients with Alzheimer's Dementia - An Explorative Intervention Study. Current Alzheimer research. 2018:15(12):1106-1113. doi: 10.2174/1389200219666180813144834. Epub [PubMed PMID: 30101706]
Adlimoghaddam A, Neuendorff M, Roy B, Albensi BC. A review of clinical treatment considerations of donepezil in severe Alzheimer's disease. CNS neuroscience & therapeutics. 2018 Oct:24(10):876-888. doi: 10.1111/cns.13035. Epub 2018 Jul 29 [PubMed PMID: 30058285]
Kumar A, Gupta V, Sharma S. Donepezil. StatPearls. 2024 Jan:(): [PubMed PMID: 30020629]
Kandiah N, Pai MC, Senanarong V, Looi I, Ampil E, Park KW, Karanam AK, Christopher S. Rivastigmine: the advantages of dual inhibition of acetylcholinesterase and butyrylcholinesterase and its role in subcortical vascular dementia and Parkinson's disease dementia. Clinical interventions in aging. 2017:12():697-707. doi: 10.2147/CIA.S129145. Epub 2017 Apr 18 [PubMed PMID: 28458525]
Kalola UK, Nguyen H. Galantamine. StatPearls. 2024 Jan:(): [PubMed PMID: 34662060]
Khoury R, Grysman N, Gold J, Patel K, Grossberg GT. The role of 5 HT6-receptor antagonists in Alzheimer's disease: an update. Expert opinion on investigational drugs. 2018 Jun:27(6):523-533. doi: 10.1080/13543784.2018.1483334. Epub 2018 Jun 18 [PubMed PMID: 29848076]
Cummings J, Lee G, Nahed P, Kambar MEZN, Zhong K, Fonseca J, Taghva K. Alzheimer's disease drug development pipeline: 2022. Alzheimer's & dementia (New York, N. Y.). 2022:8(1):e12295. doi: 10.1002/trc2.12295. Epub 2022 May 4 [PubMed PMID: 35516416]
Padda IS, Parmar M. Aducanumab. StatPearls. 2024 Jan:(): [PubMed PMID: 34424635]
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in Early Alzheimer's Disease. The New England journal of medicine. 2023 Jan 5:388(1):9-21. doi: 10.1056/NEJMoa2212948. Epub 2022 Nov 29 [PubMed PMID: 36449413]
Rashad A, Rasool A, Shaheryar M, Sarfraz A, Sarfraz Z, Robles-Velasco K, Cherrez-Ojeda I. Donanemab for Alzheimer's Disease: A Systematic Review of Clinical Trials. Healthcare (Basel, Switzerland). 2022 Dec 22:11(1):. doi: 10.3390/healthcare11010032. Epub 2022 Dec 22 [PubMed PMID: 36611492]
Sperling RA, Jack CR Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P, Carrillo MC, Thies W, Bednar MM, Black RS, Brashear HR, Grundman M, Siemers ER, Feldman HH, Schindler RJ. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011 Jul:7(4):367-85. doi: 10.1016/j.jalz.2011.05.2351. Epub [PubMed PMID: 21784348]
Grossberg GT, Kohegyi E, Mergel V, Josiassen MK, Meulien D, Hobart M, Slomkowski M, Baker RA, McQuade RD, Cummings JL. Efficacy and Safety of Brexpiprazole for the Treatment of Agitation in Alzheimer's Dementia: Two 12-Week, Randomized, Double-Blind, Placebo-Controlled Trials. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2020 Apr:28(4):383-400. doi: 10.1016/j.jagp.2019.09.009. Epub 2019 Oct 1 [PubMed PMID: 31708380]
Geldmacher DS, Whitehouse PJ Jr. Differential diagnosis of Alzheimer's disease. Neurology. 1997 May:48(5 Suppl 6):S2-9 [PubMed PMID: 9153154]
Outeiro TF, Koss DJ, Erskine D, Walker L, Kurzawa-Akanbi M, Burn D, Donaghy P, Morris C, Taylor JP, Thomas A, Attems J, McKeith I. Dementia with Lewy bodies: an update and outlook. Molecular neurodegeneration. 2019 Jan 21:14(1):5. doi: 10.1186/s13024-019-0306-8. Epub 2019 Jan 21 [PubMed PMID: 30665447]
Leroy M, Bertoux M, Skrobala E, Mode E, Adnet-Bonte C, Le Ber I, Bombois S, Cassagnaud P, Chen Y, Deramecourt V, Lebert F, Mackowiak MA, Sillaire AR, Wathelet M, Pasquier F, Lebouvier T, Méotis network. Characteristics and progression of patients with frontotemporal dementia in a regional memory clinic network. Alzheimer's research & therapy. 2021 Jan 8:13(1):19. doi: 10.1186/s13195-020-00753-9. Epub 2021 Jan 8 [PubMed PMID: 33419472]
Kuo YT, Li CY, Sung JM, Chang CC, Wang JD, Sun CY, Wu JL, Chang YT. Risk of dementia in patients with end-stage renal disease under maintenance dialysis-a nationwide population-based study with consideration of competing risk of mortality. Alzheimer's research & therapy. 2019 Apr 9:11(1):31. doi: 10.1186/s13195-019-0486-z. Epub 2019 Apr 9 [PubMed PMID: 30967155]
Barnes J, Dickerson BC, Frost C, Jiskoot LC, Wolk D, van der Flier WM. Alzheimer's disease first symptoms are age dependent: Evidence from the NACC dataset. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015 Nov:11(11):1349-57. doi: 10.1016/j.jalz.2014.12.007. Epub 2015 Apr 24 [PubMed PMID: 25916562]
Graff-Radford J, Yong KXX, Apostolova LG, Bouwman FH, Carrillo M, Dickerson BC, Rabinovici GD, Schott JM, Jones DT, Murray ME. New insights into atypical Alzheimer's disease in the era of biomarkers. The Lancet. Neurology. 2021 Mar:20(3):222-234. doi: 10.1016/S1474-4422(20)30440-3. Epub [PubMed PMID: 33609479]
Rajaram Manoharan SVR, Munakomi S. Posterior Cortical Atrophy. StatPearls. 2024 Jan:(): [PubMed PMID: 35593860]
Marshall CR, Hardy CJD, Volkmer A, Russell LL, Bond RL, Fletcher PD, Clark CN, Mummery CJ, Schott JM, Rossor MN, Fox NC, Crutch SJ, Rohrer JD, Warren JD. Primary progressive aphasia: a clinical approach. Journal of neurology. 2018 Jun:265(6):1474-1490. doi: 10.1007/s00415-018-8762-6. Epub 2018 Feb 1 [PubMed PMID: 29392464]
Barnes J, Bartlett JW, Wolk DA, van der Flier WM, Frost C. Disease Course Varies According to Age and Symptom Length in Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2018:64(2):631-642. doi: 10.3233/JAD-170841. Epub [PubMed PMID: 29914016]
Devanand DP. Behavioral complications and their treatment in Alzheimer's disease. Geriatrics. 1997 Sep:52 Suppl 2():S37-9 [PubMed PMID: 9307585]
Burgio L. Interventions for the behavioral complications of Alzheimer's disease: behavioral approaches. International psychogeriatrics. 1996:8 Suppl 1():45-52 [PubMed PMID: 8934265]
Kouloutbani K, Karteroliotis K, Politis A. [The effect of physical activity on dementia]. Psychiatrike = Psychiatriki. 2019 Apr-Jun:30(2):142-155. doi: 10.22365/jpsych.2019.302.142. Epub [PubMed PMID: 31425142]
Ginis KA, Heisz J, Spence JC, Clark IB, Antflick J, Ardern CI, Costas-Bradstreet C, Duggan M, Hicks AL, Latimer-Cheung AE, Middleton L, Nylen K, Paterson DH, Pelletier C, Rotondi MA. Formulation of evidence-based messages to promote the use of physical activity to prevent and manage Alzheimer's disease. BMC public health. 2017 Feb 17:17(1):209. doi: 10.1186/s12889-017-4090-5. Epub 2017 Feb 17 [PubMed PMID: 28212648]
Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada's Physical Activity Guidelines. The international journal of behavioral nutrition and physical activity. 2010 May 11:7():38. doi: 10.1186/1479-5868-7-38. Epub 2010 May 11 [PubMed PMID: 20459782]
Surr CA,Gates C,Irving D,Oyebode J,Smith SJ,Parveen S,Drury M,Dennison A, Effective Dementia Education and Training for the Health and Social Care Workforce: A Systematic Review of the Literature. Review of educational research. 2017 Oct; [PubMed PMID: 28989194]
Jackson M, Pelone F, Reeves S, Hassenkamp AM, Emery C, Titmarsh K, Greenwood N. Interprofessional education in the care of people diagnosed with dementia and their carers: a systematic review. BMJ open. 2016 Aug 16:6(8):e010948. doi: 10.1136/bmjopen-2015-010948. Epub 2016 Aug 16 [PubMed PMID: 27531724]