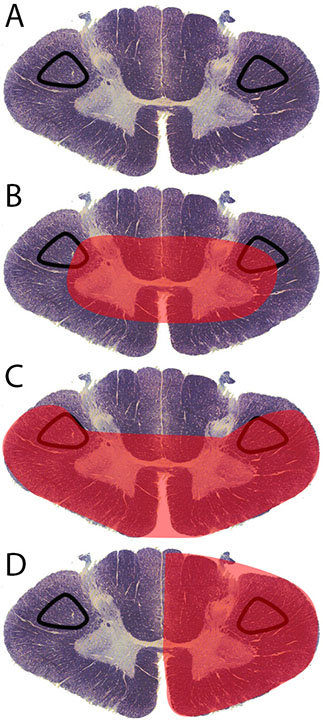
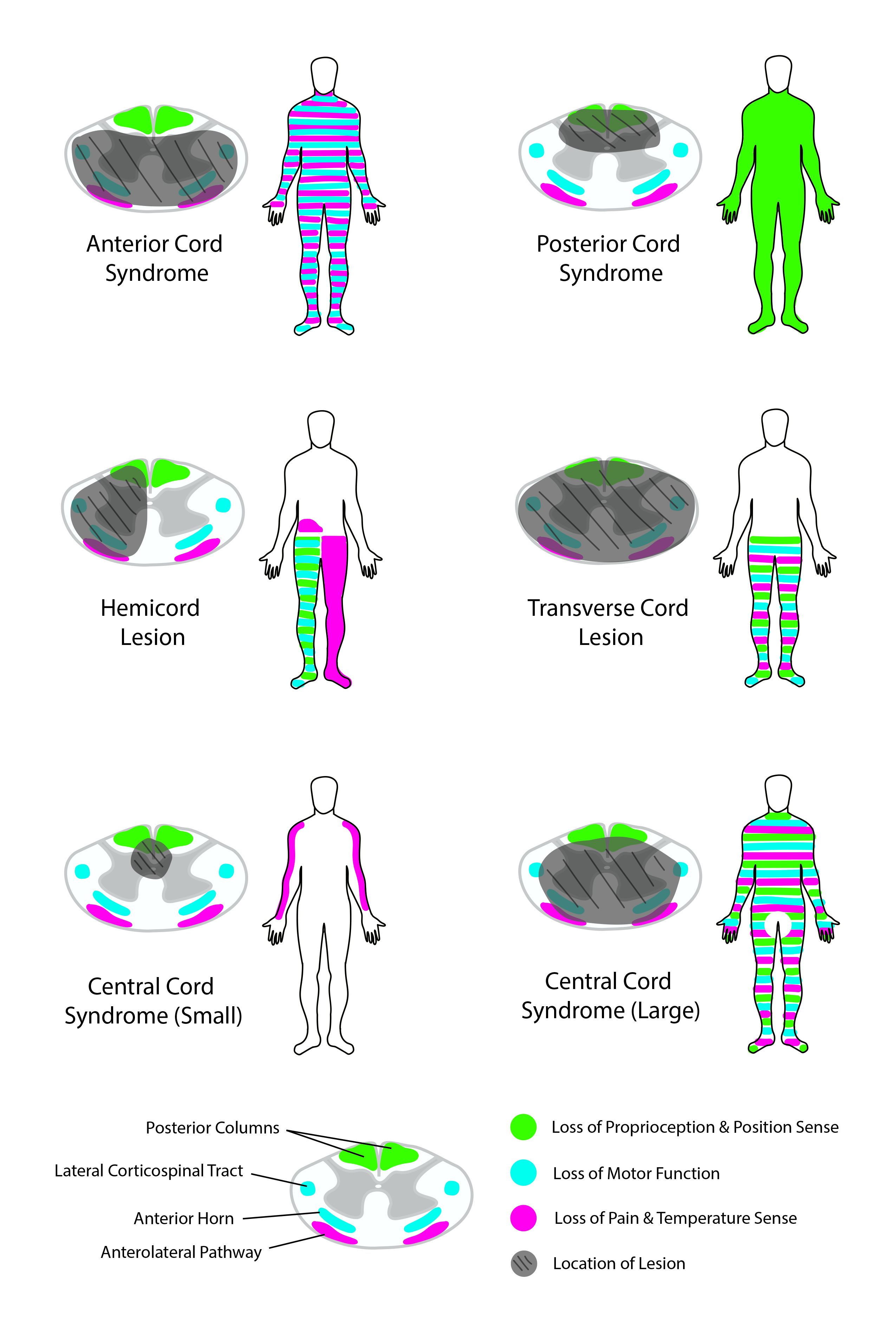
[1]
Klakeel M, Thompson J, Srinivasan R, McDonald F. Anterior spinal cord syndrome of unknown etiology. Proceedings (Baylor University. Medical Center). 2015 Jan:28(1):85-7
[PubMed PMID: 25552812]
[2]
Yogendranathan N, Herath HMMTB, Jayamali WD, Matthias AT, Pallewatte A, Kulatunga A. A case of anterior spinal cord syndrome in a patient with unruptured thoracic aortic aneurysm with a mural thrombus. BMC cardiovascular disorders. 2018 Mar 5:18(1):48. doi: 10.1186/s12872-018-0786-4. Epub 2018 Mar 5
[PubMed PMID: 29506472]
Level 3 (low-level) evidence
[3]
McKinley W, Hills A, Sima A. Posterior cord syndrome: Demographics and rehabilitation outcomes. The journal of spinal cord medicine. 2021 Mar:44(2):241-246. doi: 10.1080/10790268.2019.1585135. Epub 2019 Apr 2
[PubMed PMID: 30939076]
[4]
Piffaretti G, Bonardelli S, Bellosta R, Mariscalco G, Lomazzi C, Tolenaar JL, Zanotti C, Guadrini C, Sarcina A, Castelli P, Trimarchi S. Spinal cord ischemia after simultaneous and sequential treatment of multilevel aortic disease. The Journal of thoracic and cardiovascular surgery. 2014 Oct:148(4):1435-1442.e1. doi: 10.1016/j.jtcvs.2014.02.062. Epub 2014 Feb 26
[PubMed PMID: 24698563]
[5]
Robertson CE, Brown RD Jr, Wijdicks EF, Rabinstein AA. Recovery after spinal cord infarcts: long-term outcome in 115 patients. Neurology. 2012 Jan 10:78(2):114-21. doi: 10.1212/WNL.0b013e31823efc93. Epub 2011 Dec 28
[PubMed PMID: 22205760]
[6]
Messé SR, Bavaria JE, Mullen M, Cheung AT, Davis R, Augoustides JG, Gutsche J, Woo EY, Szeto WY, Pochettino A, Woo YJ, Kasner SE, McGarvey M. Neurologic outcomes from high risk descending thoracic and thoracoabdominal aortic operations in the era of endovascular repair. Neurocritical care. 2008:9(3):344-51. doi: 10.1007/s12028-008-9104-9. Epub
[PubMed PMID: 18483880]
[7]
Joo JB, Cummings AJ. Acute thoracoabdominal aortic dissection presenting as painless, transient paralysis of the lower extremities: a case report. The Journal of emergency medicine. 2000 Nov:19(4):333-7
[PubMed PMID: 11074326]
Level 3 (low-level) evidence
[8]
Greig D, Zoller S, Sheppard WL, Park DY. Intermittent and Transient Hypotension-related Anterior Cord Syndrome following Elective Cervical Spine Surgery: A Case Report. Journal of orthopaedic case reports. 2021 Mar:11(3):21-24. doi: 10.13107/jocr.2021.v11.i03.2070. Epub
[PubMed PMID: 34239823]
Level 3 (low-level) evidence
[9]
Schneider GS. Anterior spinal cord syndrome after initiation of treatment with atenolol. The Journal of emergency medicine. 2010 Jun:38(5):e49-52. doi: 10.1016/j.jemermed.2007.08.061. Epub 2008 Jul 2
[PubMed PMID: 18597977]
[10]
Datta AK, Mukherjee A, Ray BK, Biswas A. Anti-MOG antibody associated long segment myelitis presenting as anterior cord syndrome. BMJ case reports. 2021 May 19:14(5):. doi: 10.1136/bcr-2020-240055. Epub 2021 May 19
[PubMed PMID: 34011662]
Level 3 (low-level) evidence
[11]
Padgett M, Abi-Jaoudeh N, Benn BS, Rahimian R, Nelson K. Anterior Cord Syndrome after Embolization for Malignant Hemoptysis. Seminars in interventional radiology. 2019 Jun:36(2):111-116. doi: 10.1055/s-0039-1688424. Epub 2019 May 22
[PubMed PMID: 31123382]
[12]
Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine. 1989 Sep:68(5):282-92
[PubMed PMID: 2677596]
Level 3 (low-level) evidence
[13]
Qureshi AI, Afzal MR, Suri MFK. A Population-Based Study of the Incidence of Acute Spinal Cord Infarction. Journal of vascular and interventional neurology. 2017 Jun:9(4):44-48
[PubMed PMID: 28702119]
[14]
Weidauer S, Nichtweiss M, Lanfermann H, Zanella FE. Spinal cord infarction: MR imaging and clinical features in 16 cases. Neuroradiology. 2002 Oct:44(10):851-7
[PubMed PMID: 12389137]
Level 3 (low-level) evidence
[15]
Foo D, Rossier AB. Anterior spinal artery syndrome and its natural history. Paraplegia. 1983 Feb:21(1):1-10
[PubMed PMID: 6835686]
[16]
Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Archives of neurology. 2006 Aug:63(8):1113-20
[PubMed PMID: 16908737]
[17]
Vargas MI, Gariani J, Sztajzel R, Barnaure-Nachbar I, Delattre BM, Lovblad KO, Dietemann JL. Spinal cord ischemia: practical imaging tips, pearls, and pitfalls. AJNR. American journal of neuroradiology. 2015 May:36(5):825-30. doi: 10.3174/ajnr.A4118. Epub 2014 Oct 16
[PubMed PMID: 25324492]
[18]
Masson C, Pruvo JP, Meder JF, Cordonnier C, Touzé E, De La Sayette V, Giroud M, Mas JL, Leys D, Study Group on Spinal Cord Infarction of the French Neurovascular Society. Spinal cord infarction: clinical and magnetic resonance imaging findings and short term outcome. Journal of neurology, neurosurgery, and psychiatry. 2004 Oct:75(10):1431-5
[PubMed PMID: 15377691]
[19]
Santillan A, Goldberg JL, Carnevale JA, Kirnaz S, Hartl R, Knopman J. Anterior spinal artery syndrome caused by thoracic disc herniation. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2020 Jul:77():211-212. doi: 10.1016/j.jocn.2020.05.040. Epub 2020 May 11
[PubMed PMID: 32409217]
[20]
Cheung AT, Pochettino A, McGarvey ML, Appoo JJ, Fairman RM, Carpenter JP, Moser WG, Woo EY, Bavaria JE. Strategies to manage paraplegia risk after endovascular stent repair of descending thoracic aortic aneurysms. The Annals of thoracic surgery. 2005 Oct:80(4):1280-8; discussion 1288-9
[PubMed PMID: 16181855]
[21]
McGarvey ML, Cheung AT, Szeto W, Messe SR. Management of neurologic complications of thoracic aortic surgery. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2007 Aug:24(4):336-43
[PubMed PMID: 17938602]
[22]
Jankovic J, Rey Bataillard V, Mercier N, Bonvin C, Michel P. Acute ischemic myelopathy treated with intravenous thrombolysis: Four new cases and literature review. International journal of stroke : official journal of the International Stroke Society. 2019 Dec:14(9):893-897. doi: 10.1177/1747493019851289. Epub 2019 May 15
[PubMed PMID: 31092154]
Level 3 (low-level) evidence
[23]
Nedeltchev K, Loher TJ, Stepper F, Arnold M, Schroth G, Mattle HP, Sturzenegger M. Long-term outcome of acute spinal cord ischemia syndrome. Stroke. 2004 Feb:35(2):560-5
[PubMed PMID: 14726546]
[24]
Tang W, Yao L, Roetker NS, Alonso A, Lutsey PL, Steenson CC, Lederle FA, Hunter DW, Bengtson LG, Guan W, Missov E, Folsom AR. Lifetime Risk and Risk Factors for Abdominal Aortic Aneurysm in a 24-Year Prospective Study: The ARIC Study (Atherosclerosis Risk in Communities). Arteriosclerosis, thrombosis, and vascular biology. 2016 Dec:36(12):2468-2477
[PubMed PMID: 27834688]
[25]
Gawinecka J, Schönrath F, von Eckardstein A. Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss medical weekly. 2017:147():w14489. doi: 10.4414/smw.2017.14489. Epub 2017 Aug 25
[PubMed PMID: 28871571]
[26]
Elsharawy MA, Alkhadra AH, Ibrahim MF, Selim F, Hassan K, Elsaid AS, Bahnassy A. Impact of atherosclerosis risk factors on the clinical presentation of arterial occlusive disease in Arabic patients. The International journal of angiology : official publication of the International College of Angiology, Inc. 2008 Winter:17(4):203-6
[PubMed PMID: 22477450]
[27]
Gómara-Toldrà N, Sliwinski M, Dijkers MP. Physical therapy after spinal cord injury: a systematic review of treatments focused on participation. The journal of spinal cord medicine. 2014 Jul:37(4):371-9. doi: 10.1179/2045772314Y.0000000194. Epub 2014 Jan 21
[PubMed PMID: 24621042]
Level 1 (high-level) evidence
[28]
Ragnarsson KT. Medical rehabilitation of people with spinal cord injury during 40 years of academic physiatric practice. American journal of physical medicine & rehabilitation. 2012 Mar:91(3):231-42. doi: 10.1097/PHM.0b013e3182489f5e. Epub
[PubMed PMID: 22317933]