Continuing Education Activity
A vidian neurectomy is a surgical procedure involving sacrificing the vidian nerve to address refractory vasomotor rhinitis. The surgeon identifies and removes a nerve segment through endoscopic techniques, disrupting the nasal cavity's autonomic supply and reducing nasal secretions. The procedure was pioneered in 1991 and offers a more precise and less morbid alternative to traditional open approaches, yielding improved nasal symptom outcomes.
Clinicians engaging in this continuing education activity on vidian neurectomy can expect to gain advanced proficiency in patient selection, precise execution of surgical techniques, and an in-depth understanding of potential complications. This knowledge enhances their competence in managing refractory vasomotor rhinitis cases, promoting optimal outcomes and enabling informed patient decision-making. Additionally, participants will stay informed on the latest advancements and evidence-based practices in the field, contributing to their ongoing professional development.
Objectives:
Differentiate between traditional open approaches and endoscopic transnasal vidian neurectomy techniques, understanding the advantages and disadvantages of each.
Assess postoperative outcomes, systematically assessing the effectiveness of vidian neurectomy in improving nasal symptoms and overall patient satisfaction.
Identify indications for vidian neurectomy, particularly in cases of refractory vasomotor rhinitis.
Communicate and collaborate effectively with members of the interprofessional healthcare team to develop coordinated strategies for implementing vidian neurectomies.
Introduction
The nerve of the pterygoid canal, also known as the vidian nerve, supplies parasympathetic fibers to the nasal mucosa, palate, and lacrimal gland through the pterygopalatine ganglion. A vidian neurectomy, the sacrifice of this nerve, initially described by Golding-Wood in 1961, diminishes autonomic supply to the nasal cavity and reduces nasal secretions.[1][2]
The primary indication for a vidian neurectomy is vasomotor rhinitis, believed to stem from an imbalance between parasympathetic and sympathetic supply to the nasal mucosa. In the preendoscopic era, locating the vidian nerve was challenging, leading to suboptimal long-term outcomes and infrequent procedure utilization. Open approaches to the pterygopalatine fossa, like transantral and transpalatal exposures, were associated with significant morbidity, including ophthalmoplegia, orbital complications, and palatal fistulae.[3] In a groundbreaking development in 1991, Kamel and Zaher introduced endoscopic transnasal vidian neurectomy in cadaveric models. This innovation paved the way for modern surgical techniques, offering a more precise and less morbid approach.[4]
Clinical studies have reported improved nasal symptom outcomes with vidian neurectomy compared to medical management or alternative surgical procedures like turbinoplasty or septoplasty.[5] Despite the growing interest in vidian neurectomy, there remains limited evidence concerning its long-term results and potential complications. Further research is needed to comprehensively understand the procedure's efficacy and safety in the context of prolonged outcomes.
Anatomy and Physiology
Anatomy of the Vidian Nerve
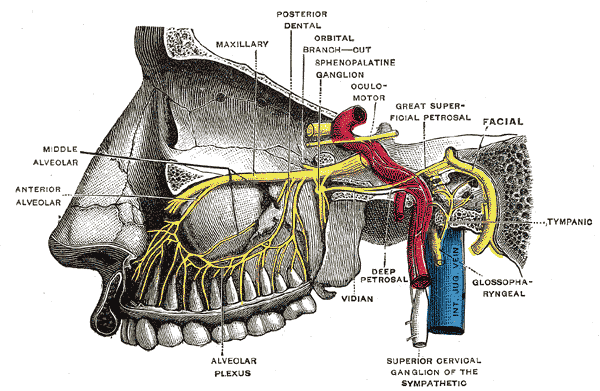
The vidian nerve is accompanied by the vidian artery and courses through the pterygoid canal (also known as the vidian canal), an osseous tunnel along the sphenoid sinus floor. The vidian nerve, originating from the convergence of parasympathetic fibers from the greater superficial petrosal nerve and sympathetic fibers from the deep petrosal nerve (derived from the internal carotid artery plexus), extends into the pterygopalatine ganglion located within the pterygopalatine fossa (see Image. Pterygopalatine Ganglion, Deep Petrosal Nerve, and Vidian Nerve). The vidian canal connects the foramen lacerum in the middle cranial fossa with the pterygopalatine fossa. This canal runs in a medial to lateral direction, traversing the sphenoid sinus floor to its funnel-shaped opening into the pterygopalatine fossa at the pterygoid "wedge."[6]
The pterygoid wedge, a valuable landmark for identification of the vidian canal, lies at the anterior junction of the pterygoid plates, which are located beneath the sphenoid sinus floor. Three openings exist along the floor of the sphenoid sinus, arranged from medial to lateral:
- Palatovaginal canal (transmitting the pharyngeal branch of the internal maxillary artery and the posterior pharyngeal nerve)
- Vidian canal
- Foramen rotundum (conveying the maxillary branch of the trigeminal nerve to the midface)
The pterygopalatine fossa, which houses the pterygopalatine ganglion, contains various nerves in addition to the vidian nerve. This fossa has an inverted pyramid shape and is located just behind the maxillary sinus. The pterygoid canal and the vidian nerve enter the fossa's posterior aspect. The maxillary division of the trigeminal nerve runs superior to the vidian nerve and the pterygopalatine ganglion and is connected to the ganglion via sensory fibers. Emanating from the ganglion are both somatic sensory and autonomic fibers, provided by the maxillary nerve (somatic sensory), the greater superficial petrosal nerve (parasympathetic), and the deep petrosal nerve (sympathetic). These branches include the greater and lesser palatine and nasopalatine nerves (sensory), the posterior nasal nerve, and other autonomic nerves to the lacrimal gland and the mucosal glands of the nose, palate, and pharynx. Knowing the location of these structures can help reduce the chances of surgical complications, such as numbness and xerophthalmia.
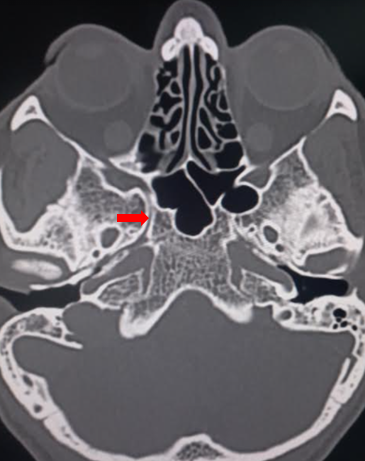
Before undertaking surgery in the area, it is crucial to understand the variations in the course and location of the vidian nerve relative to the sphenoid sinus. Radiographic studies indicate a medial to lateral course of the vidian canal from the pterygopalatine fossa to the foramen lacerum in 80% to 98% of cases (see Image. Vidian Canal).[7][8] The vidian canal is approximately 18 mm in length. The location of the vidian nerve within the sphenoid sinus exhibits significant variability, not only in its protrusion from the floor (inside the sphenoid corpus, partially protruding or connected by a bony stalk) but also in the angle formed by the floor with the nerve (flat, upsloping, downsloping, or inverted-V types).[9] Understanding these nuances is essential for ensuring surgical precision and minimizing potential complications.
Endoscopic Landmarks for the Vidian Canal
- The vidian canal is visible as an elevation along the lateral aspect of the sphenoid sinus floor.
- The opening of the vidian canal into the pterygopalatine fossa is located at the intersection between a line drawn along the posterior border of the palatine bone and a horizontal line drawn along the sphenoid sinus floor.
Surgical Relevance of the Vidian Canal
- Endoscopic management of juvenile nasopharyngeal angiofibromas
- Used as a landmark for the petrous carotid artery in expanded endonasal approaches
- Vidian neurectomy
Indications
A vidian neurectomy is indicated in the following cases:
- Refractory vasomotor rhinitis and other nonallergic rhinitis
- Perennial allergic rhinitis
- Chronic cluster headaches refractory to medical management [10]
- Chronic epiphora
- Senile nasal drip
- Crocodile tears (Bogorad syndrome) as an alternate option to tympanic neurectomy [3]
Contraindications
There are no absolute contraindications to a vidian neurectomy. Relative contraindications to this procedure are skull base defects or tumors in the pterygomaxillary region.
Equipment
Equipment required for a vidian neurectomy includes:
- Functional endoscopic sinus surgery instrument set
- Endoscopic skull base instrument set, including endoscopic ligating clips, applicators, and an endoscopic drill system
- Endoscopes (0°, 45°, and 70°)
- Image-guided navigation system
- Epinephrine-soaked neurological sponges
- Nasal packing
Personnel
For a vidian neurectomy, a coordinated team approach involving specialized nurses in rhinology/endoscopic skull base surgery, an anesthesia provider, an endoscopic skull base surgeon, and an assistant is necessary.
Preparation
In preparation for surgery, obtaining a computed tomography scan of the paranasal sinuses with 1 mm contiguous axial, coronal, and sagittal views is necessary. During surgical planning, special attention is given to the position of the vidian canal in the sphenoid sinus, its relationship with the sphenoid corpus, the thickness of the bone covering the roof of the canal, and the angle formed between the floor of the sphenoid sinus and the canal.[9]
After orotracheal intubation, the patient is positioned in a semi-Fowler position (supine with the head of the bed elevated 30 to 45 degrees) with the head in a horseshoe rest or Mayfield pin holder. A pterygopalatine ganglion block is performed transorally via the greater palatine canal. The nasal cavity is prepared and decongested with oxymetazoline or epinephrine-soaked neurological sponges.
Technique or Treatment
Numerous descriptions in the literature of approaches to the vidian nerve include transantral via a Caldwell-Luc approach, transpalatal, transseptal mucoperichondrial, and endonasal routes. With advancements in endoscopic sinus surgery in the last 3 decades, the preferred route for vidian neurectomy has become the endoscopic endonasal route, using either the transsphenoidal or transnasal approach. The transsphenoidal approach is preferable in cases with a prominent vidian canal in the sphenoid sinus floor. However, both techniques can be combined in varying degrees to trace the vidian nerve from the sphenoid sinus to the pterygopalatine ganglion.
Surgical Steps of Transnasal or Retrograde Approaches
The surgical procedure begins by infiltrating the lateral wall of the nasal cavity anterior to the posterior end of the middle turbinate with a local anesthetic containing epinephrine. Subsequently, a U-shaped, posteriorly-based flap is raised over the palatine bone, positioned posterior to the posterior fontanelle of the maxillary sinus. The surgeon then identifies the ethmoidal crest of the palatine bone, a crucial landmark for locating the sphenopalatine foramen. After pinpointing the sphenopalatine artery, it is coagulated or clipped. The mucosal flap is elevated behind the foramen onto the face of the sphenoid sinus. Removing the posteroinferior margin of the sphenopalatine foramen eliminates the medial wall of the pterygopalatine fossa. The contents of the pterygopalatine fossa are laterally displaced to expose the vidian canal. Following clear visualization and identification of the vidian nerve, a 2- to 3-mm segment is excised using a sickle knife or scissors. Finally, the mucosal flap is repositioned and supported with a small piece of Gelfoam to complete the procedure.
Surgical Steps of Transsphenoidal or Anterograde Approaches
The identification of the sphenoid sinus ostium is carried out approximately 1.2 to 1.5 cm above the choana. To ensure the preservation of sphenopalatine artery branches inferolateral, a wide sphenoidotomy is performed, and the sphenoid rostrum is subsequently removed. Attention is directed to the potential risk of injury to the posterior septal branch of the sphenopalatine artery as it courses inferomedially, traversing superior to the choana and inferior to the sphenoid os. Following the thinning of the sphenoid sinus floor, the vidian nerve is identified on the lateral aspect of the floor using a 70-degree endoscope. If not readily visible, the canal is exposed using a pricking probe, Kerrison's rongeur, or drill. Subsequently, the nerve is transected with an angled probe, and a segment is excised. In the event of significant intraoperative bleeding, nasal packing is employed.
Pitfalls in Surgery
- Misidentification of the vidian canal
- The palatovaginal and vidian canals run along the sphenoid sinus floor; their proximity can easily confuse them. The palatovaginal canal's anterior opening is just a few millimeters away from the vidian canal's opening. However, there are some distinct differences between them. The palatovaginal canal is smaller than the vidian canal, and its opening is just medial to the vidian canal. Additionally, the palatovaginal canal transmits a smaller nerve known as the posterior pharyngeal nerve.
- Misidentification of the vidian nerve
- Due to their proximity, the posterior pharyngeal nerve can be mistaken for the vidian nerve. The posterior pharyngeal nerve exits the palatovaginal canal to run across the vidian canal in a lateral direction to join the pterygopalatine ganglion in the pterygopalatine fossa, while the vidian nerve, much greater in thickness, takes a slight lateral course before it joins the pterygopalatine ganglion.
- Incomplete resection of the vidian nerve
Complications
Immediate Complications
- Postoperative bleeding
- The bleeding usually comes from branches of the sphenopalatine artery, which can be controlled with nasal packing or cautery.
Long Term Complications
- Dry eye
- This is the most commonly reported complication, occurring between 35% to 72% of the time.
- Xerophthalmia is significantly more likely to occur after transsphenoidal approaches.
- Most studies reported the resolution of dry eyes within 1 to 5 months postoperatively.
- Palatal, gingival, and cheek numbness
- This occurs in 6.27% of patients.
- The incidence is higher with the pterygopalatine approach.
- Nasal crusting and dryness [11]
- This occurs in 3.7% of patients.
Clinical Significance
Surgical management of refractory rhinitis is still evolving, aiming to eliminate or substantially reduce parasympathetic innervation to the nasal mucosa. Endoscopic vidian neurectomy has proved to be an effective procedure with long-term (2 to 5 years) control of rhinorrhea.[11] To decrease complications of the procedure, particularly xerophthalmia and palate or cheek numbness, newer techniques, including posterior nasal neurectomy, are being studied.[12]
The posterior nasal nerve, a parasympathetic branch off the pterygopalatine ganglion, provides sensory and autonomic innervation to the nasal mucosa.[13] Sectioning of this nerve via an endoscopic approach provides symptomatic relief comparable to vidian neurectomy. However, with a lower likelihood of long-term complications due to injury to other nerves within the pterygopalatine fossa.[14] Because the posterior nasal nerve enters the nasal cavity just posterior to the middle and inferior turbinates, it is easily accessible endoscopically. In-office endoscopic ablation techniques are becoming more popular. The ablation modality most commonly employed is cryogenic, which appears to be as effective as vidian neurectomy but without a requirement for general anesthesia or as high a likelihood of causing xerophthalmia or cheek and palate numbness.[15][16] Radiofrequency and LASER ablation are also emerging as effective, safe alternative options.[17][18]
Enhancing Healthcare Team Outcomes
Concerning a vidian neurectomy, a collaborative healthcare team is essential for providing patient-centered care and optimizing outcomes. Surgeons and advanced practitioners must possess advanced endoscopic skills to identify and manipulate nasal structures, particularly the vidian nerve. Their responsibilities encompass accurate patient selection, meticulous execution of the surgical procedure, and effective postoperative management. Nurses play a crucial role in preoperative patient education, perioperative care, and postoperative monitoring, ensuring seamless transitions between different phases of care. Pharmacists contribute by reviewing medication regimens, managing pain effectively, providing guidance on potential drug interactions, and enhancing patient safety.Interprofessional communication is fundamental to the success of a vidian neurectomy. Physicians, advanced practitioners, nurses, pharmacists, and other health professionals must communicate clearly to share insights, discuss patient progress, and promptly address concerns. A cohesive approach to care coordination is vital, with each team member understanding their responsibilities and collaborating to implement a comprehensive care plan. This synergy ensures a patient-centric focus, minimizes the risk of complications, and optimizes team performance, contributing to enhanced patient safety and overall positive outcomes.