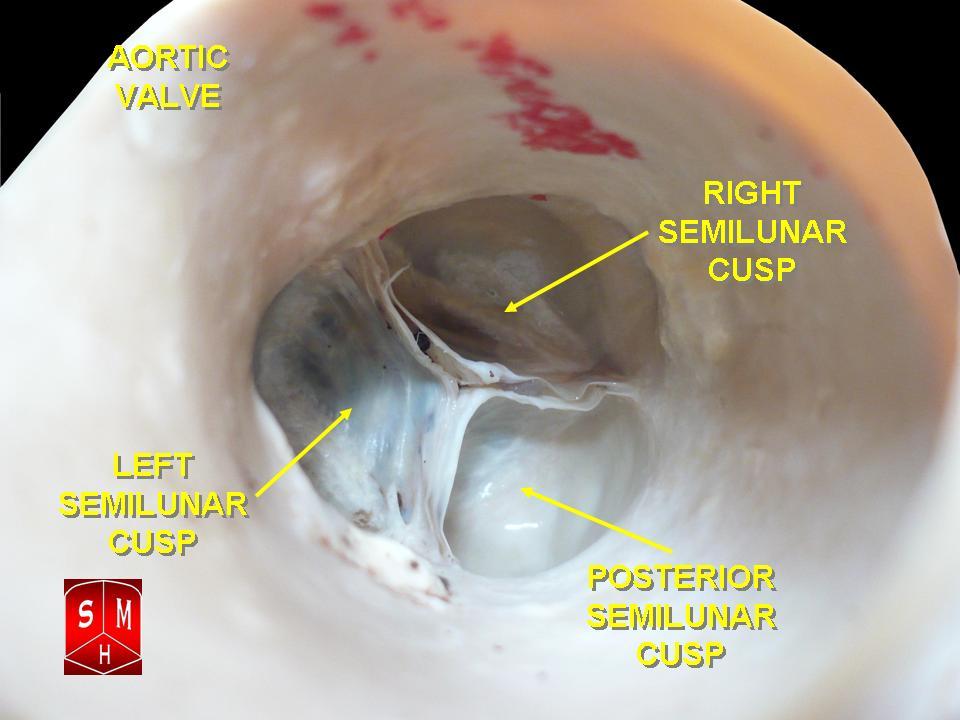
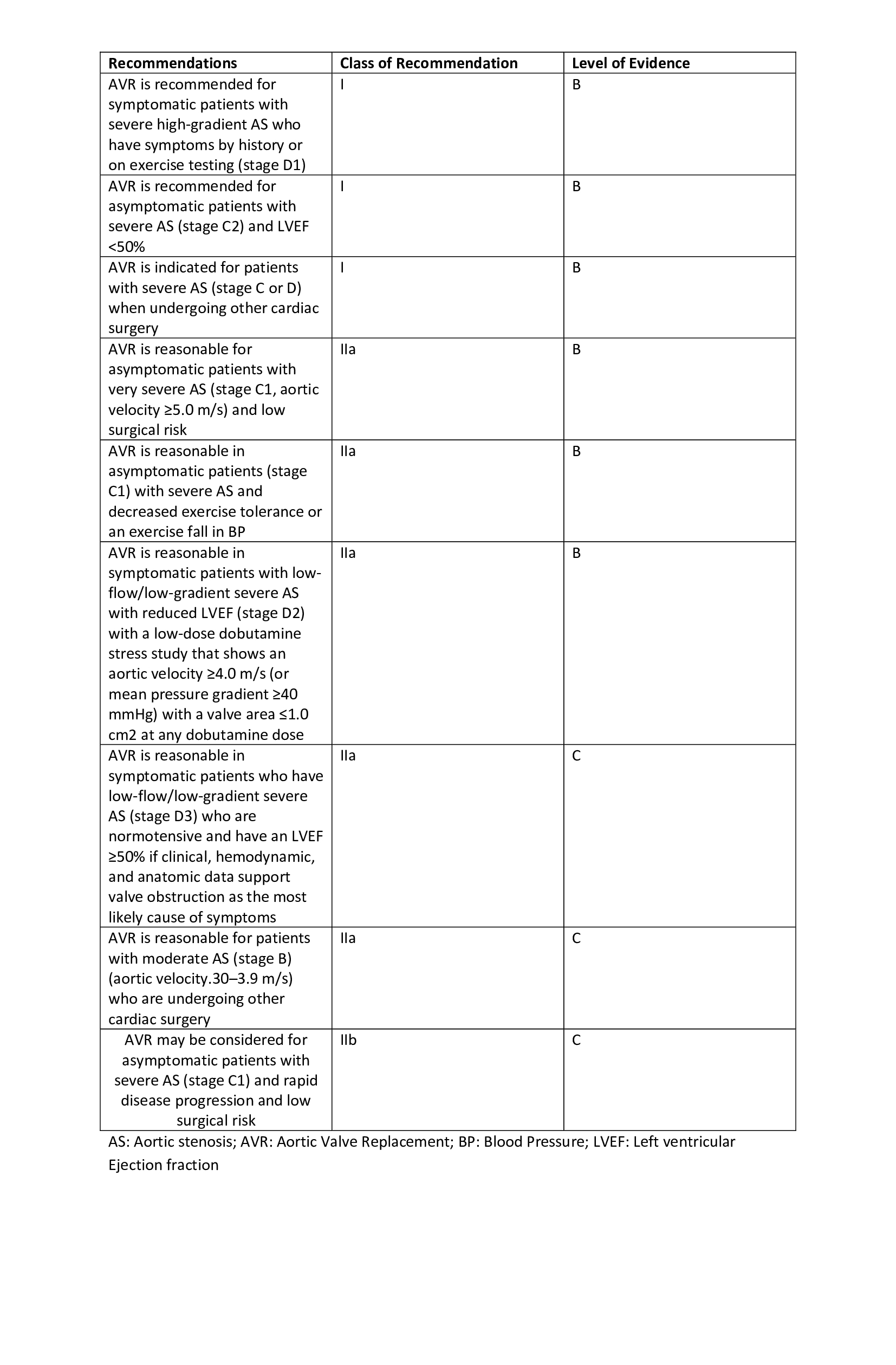
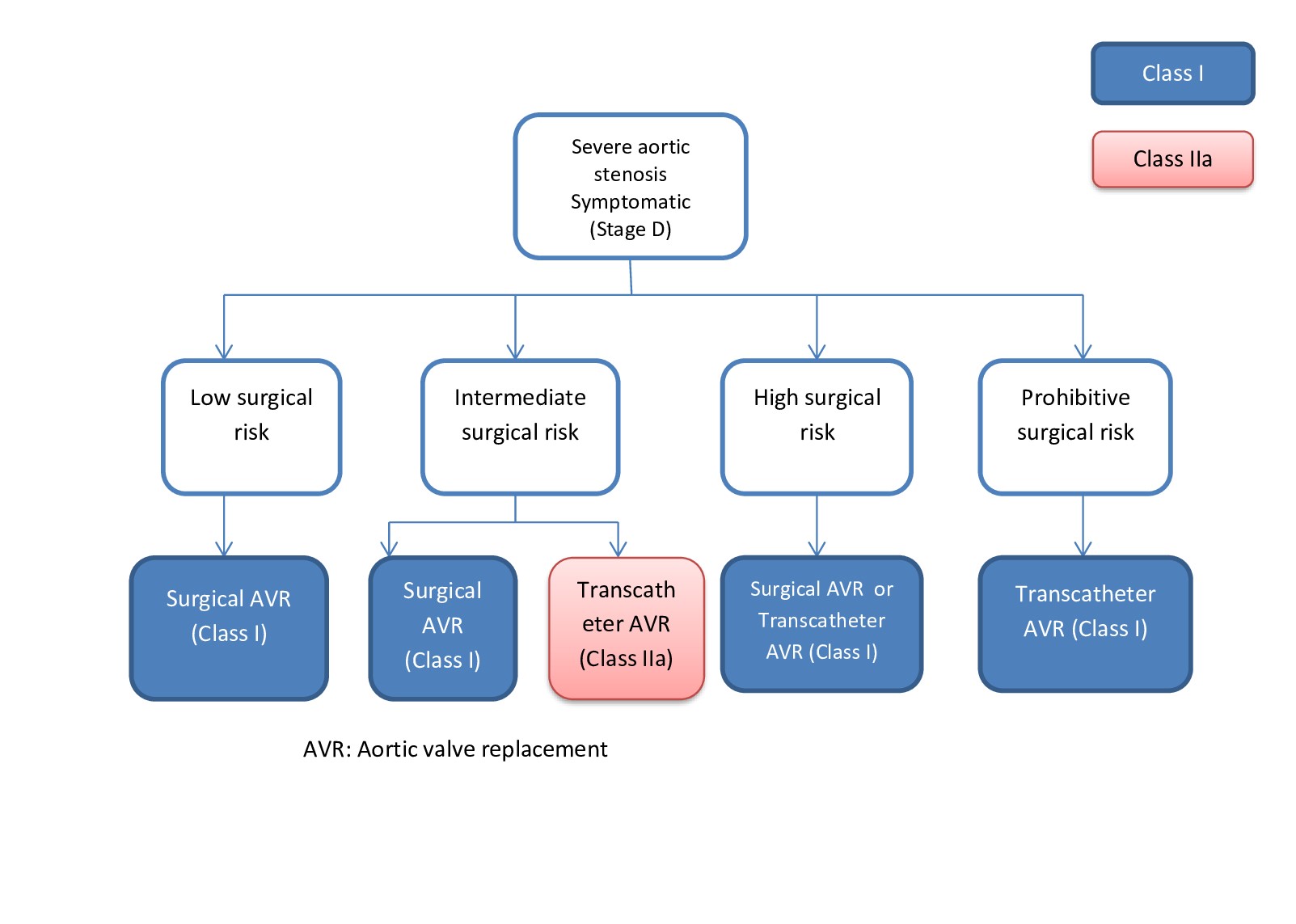
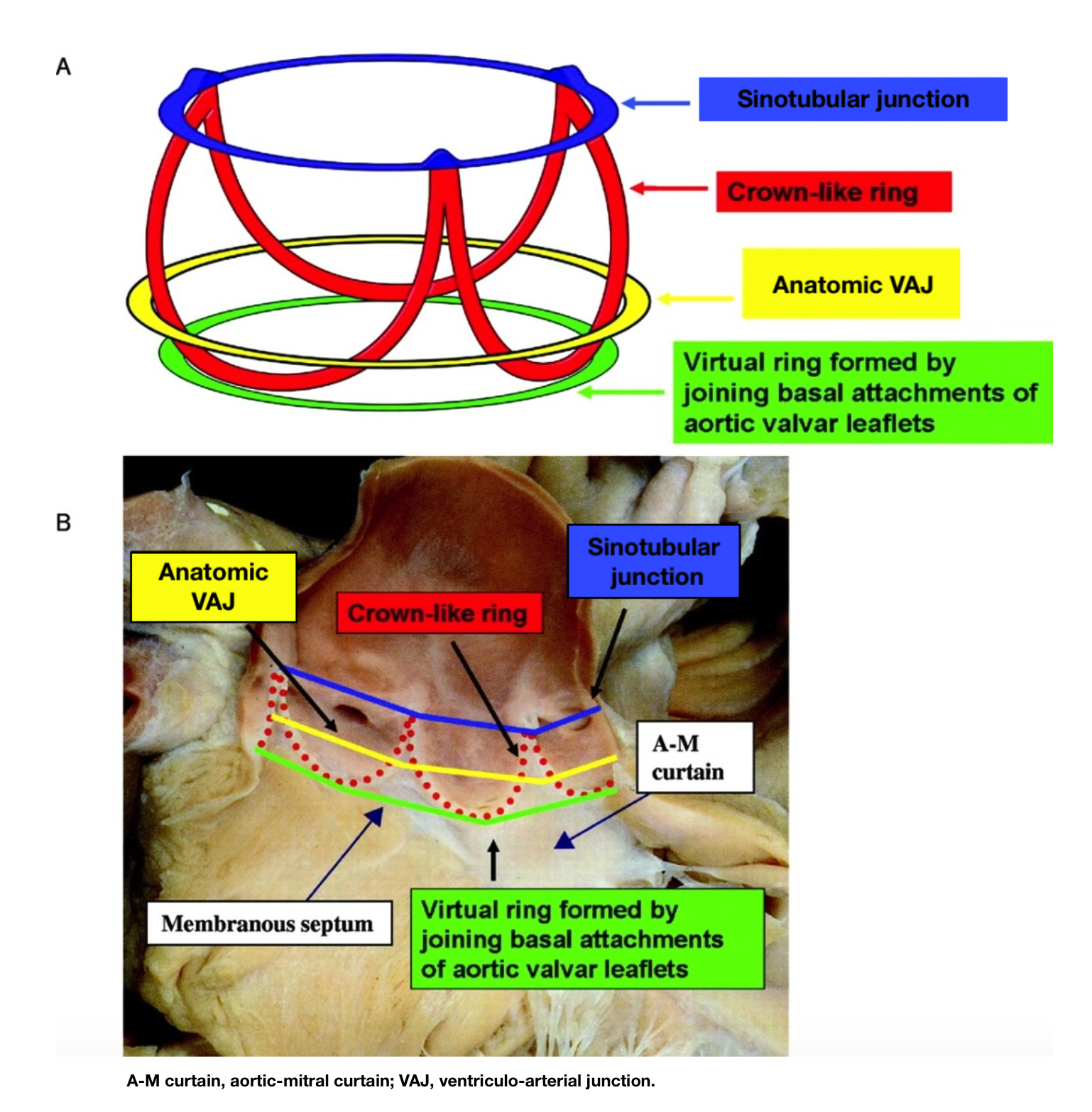
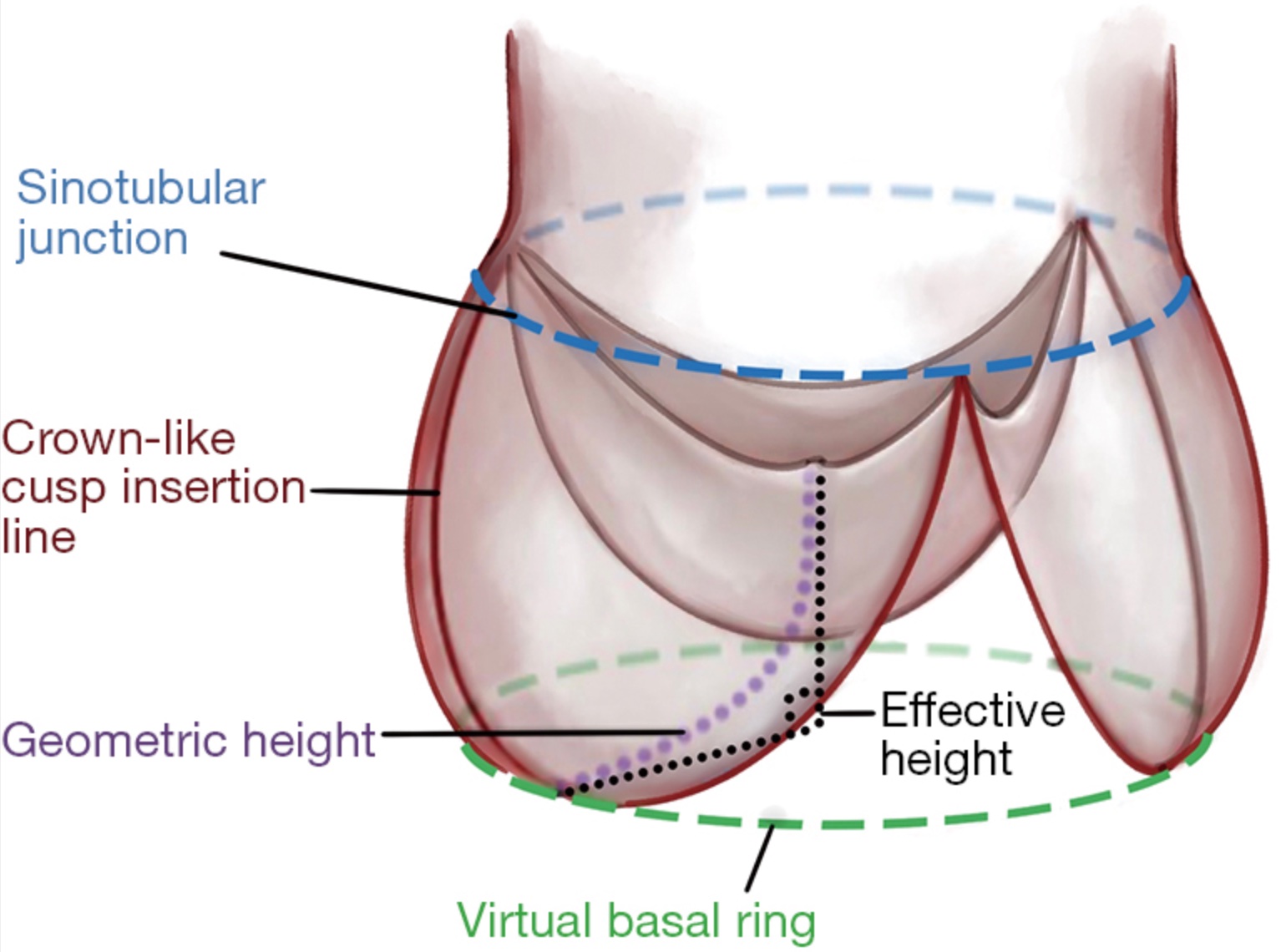
[1]
Notenboom ML, Van Hoof L, Schuermans A, Takkenberg JJM, Rega FR, Taverne YJHJ. Aortic Valve Embryology, Mechanobiology, and Second Messenger Pathways: Implications for Clinical Practice. Journal of cardiovascular development and disease. 2024 Feb 1:11(2):. doi: 10.3390/jcdd11020049. Epub 2024 Feb 1
[PubMed PMID: 38392263]
[2]
Yacoub MH, Cohn LH. Novel approaches to cardiac valve repair: from structure to function: Part II. Circulation. 2004 Mar 9:109(9):1064-72
[PubMed PMID: 15007015]
[3]
El Khoury G, de Kerchove L. Principles of aortic valve repair. The Journal of thoracic and cardiovascular surgery. 2013 Mar:145(3 Suppl):S26-9. doi: 10.1016/j.jtcvs.2012.11.071. Epub 2012 Dec 20
[PubMed PMID: 23260436]
[4]
Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. European heart journal. 2003 Jul:24(13):1231-43
[PubMed PMID: 12831818]
Level 3 (low-level) evidence
[5]
Carr JA, Savage EB. Aortic valve repair for aortic insufficiency in adults: a contemporary review and comparison with replacement techniques. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2004 Jan:25(1):6-15
[PubMed PMID: 14690726]
[6]
Aicher D, Fries R, Rodionycheva S, Schmidt K, Langer F, Schäfers HJ. Aortic valve repair leads to a low incidence of valve-related complications. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2010 Jan:37(1):127-32. doi: 10.1016/j.ejcts.2009.06.021. Epub 2009 Jul 29
[PubMed PMID: 19643618]
[7]
Anderson RH. Clinical anatomy of the aortic root. Heart (British Cardiac Society). 2000 Dec:84(6):670-3
[PubMed PMID: 11083753]
[8]
Underwood MJ, El Khoury G, Deronck D, Glineur D, Dion R. The aortic root: structure, function, and surgical reconstruction. Heart (British Cardiac Society). 2000 Apr:83(4):376-80
[PubMed PMID: 10722531]
[9]
Crooke PS, Beavan LA, Griffin CD, Mazzitelli D, Rankin JS. A geometric model of the normal human aortic root and design of a fully anatomic aortic root graft. Innovations (Philadelphia, Pa.). 2015 Jan-Feb:10(1):57-62. doi: 10.1097/IMI.0000000000000125. Epub
[PubMed PMID: 25628255]
[10]
Kim M, Roman MJ, Cavallini MC, Schwartz JE, Pickering TG, Devereux RB. Effect of hypertension on aortic root size and prevalence of aortic regurgitation. Hypertension (Dallas, Tex. : 1979). 1996 Jul:28(1):47-52
[PubMed PMID: 8675263]
[11]
de Kerchove L, El Khoury G. Anatomy and pathophysiology of the ventriculo-aortic junction: implication in aortic valve repair surgery. Annals of cardiothoracic surgery. 2013 Jan:2(1):57-64. doi: 10.3978/j.issn.2225-319X.2012.12.05. Epub
[PubMed PMID: 23977560]
[12]
Khelil N, Sleilaty G, Palladino M, Fouda M, Escande R, Debauchez M, Di Centa I, Lansac E. Surgical anatomy of the aortic annulus: landmarks for external annuloplasty in aortic valve repair. The Annals of thoracic surgery. 2015 Apr:99(4):1220-6. doi: 10.1016/j.athoracsur.2014.12.034. Epub 2015 Feb 27
[PubMed PMID: 25728963]
[13]
de Kerchove L, Jashari R, Boodhwani M, Duy KT, Lengelé B, Gianello P, Mosala Nezhad Z, Astarci P, Noirhomme P, El Khoury G. Surgical anatomy of the aortic root: implication for valve-sparing reimplantation and aortic valve annuloplasty. The Journal of thoracic and cardiovascular surgery. 2015 Feb:149(2):425-33. doi: 10.1016/j.jtcvs.2014.09.042. Epub 2014 Sep 18
[PubMed PMID: 25439782]
[14]
Schäfers HJ, Schmied W, Marom G, Aicher D. Cusp height in aortic valves. The Journal of thoracic and cardiovascular surgery. 2013 Aug:146(2):269-74. doi: 10.1016/j.jtcvs.2012.06.053. Epub 2012 Jul 31
[PubMed PMID: 22853942]
[15]
Giebels C, Ehrlich T, Schäfers HJ. Aortic root remodeling. Annals of cardiothoracic surgery. 2023 Jul 31:12(4):369-376. doi: 10.21037/acs-2023-avs2-12. Epub 2023 Jul 6
[PubMed PMID: 37554714]
[16]
Gillinov AM, Mihaljevic T, Blackstone EH, George K, Svensson LG, Nowicki ER, Sabik JF 3rd, Houghtaling PL, Griffin B. Should patients with severe degenerative mitral regurgitation delay surgery until symptoms develop? The Annals of thoracic surgery. 2010 Aug:90(2):481-8. doi: 10.1016/j.athoracsur.2010.03.101. Epub
[PubMed PMID: 20667334]
[17]
American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, Bonow RO, Carabello BA, Kanu C, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006 Aug 1:114(5):e84-231
[PubMed PMID: 16880336]
Level 1 (high-level) evidence
[18]
Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, Society for Vascular Medicine. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010 Apr 6:121(13):e266-369. doi: 10.1161/CIR.0b013e3181d4739e. Epub 2010 Mar 16
[PubMed PMID: 20233780]
Level 3 (low-level) evidence
[19]
Svensson LG, Adams DH, Bonow RO, Kouchoukos NT, Miller DC, O'Gara PT, Shahian DM, Schaff HV, Akins CW, Bavaria J, Blackstone EH, David TE, Desai ND, Dewey TM, D'Agostino RS, Gleason TG, Harrington KB, Kodali S, Kapadia S, Leon MB, Lima B, Lytle BW, Mack MJ, Reece TB, Reiss GR, Roselli E, Smith CR, Thourani VH, Tuzcu EM, Webb J, Williams MR. Aortic valve and ascending aorta guidelines for management and quality measures: executive summary. The Annals of thoracic surgery. 2013 Apr:95(4):1491-505. doi: 10.1016/j.athoracsur.2012.12.027. Epub 2013 Jan 2
[PubMed PMID: 23291103]
Level 2 (mid-level) evidence
[20]
Sharma V, Suri RM, Dearani JA, Burkhart HM, Park SJ, Joyce LD, Li Z, Schaff HV. Expanding relevance of aortic valve repair-is earlier operation indicated? The Journal of thoracic and cardiovascular surgery. 2014 Jan:147(1):100-7. doi: 10.1016/j.jtcvs.2013.08.015. Epub 2013 Sep 29
[PubMed PMID: 24084289]
[21]
Boodhwani M, de Kerchove L, Glineur D, Poncelet A, Rubay J, Astarci P, Verhelst R, Noirhomme P, El Khoury G. Repair-oriented classification of aortic insufficiency: impact on surgical techniques and clinical outcomes. The Journal of thoracic and cardiovascular surgery. 2009 Feb:137(2):286-94. doi: 10.1016/j.jtcvs.2008.08.054. Epub 2008 Dec 27
[PubMed PMID: 19185138]
Level 2 (mid-level) evidence
[22]
Aicher D, Kunihara T, Abou Issa O, Brittner B, Gräber S, Schäfers HJ. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation. 2011 Jan 18:123(2):178-85. doi: 10.1161/CIRCULATIONAHA.109.934679. Epub 2011 Jan 3
[PubMed PMID: 21200006]
[23]
Boodhwani M, de Kerchove L, Glineur D, Rubay J, Vanoverschelde JL, Noirhomme P, El Khoury G. Repair of regurgitant bicuspid aortic valves: a systematic approach. The Journal of thoracic and cardiovascular surgery. 2010 Aug:140(2):276-284.e1. doi: 10.1016/j.jtcvs.2009.11.058. Epub 2010 May 20
[PubMed PMID: 20488466]
Level 1 (high-level) evidence
[24]
Sadovnick AD, Armstrong H, Rice GP, Bulman D, Hashimoto L, Paty DW, Hashimoto SA, Warren S, Hader W, Murray TJ. A population-based study of multiple sclerosis in twins: update. Annals of neurology. 1993 Mar:33(3):281-5
[PubMed PMID: 8498811]
[25]
Vendramin I, Bortolotti U, De Manna DN, Lechiancole A, Sponga S, Livi U. Combined Replacement of Aortic Valve and Ascending Aorta-A 70-Year Evolution of Surgical Techniques. Aorta (Stamford, Conn.). 2021 Jun:9(3):118-123. doi: 10.1055/s-0041-1729913. Epub 2021 Oct 11
[PubMed PMID: 34634836]
[26]
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W, ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. European heart journal. 2022 Feb 12:43(7):561-632. doi: 10.1093/eurheartj/ehab395. Epub
[PubMed PMID: 34453165]
[27]
Boodhwani M, de Kerchove L, Glineur D, Rubay J, Vanoverschelde JL, Van Dyck M, Noirhomme P, El Khoury G. Aortic valve repair with ascending aortic aneurysms: associated lesions and adjunctive techniques. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2011 Aug:40(2):424-8. doi: 10.1016/j.ejcts.2010.11.053. Epub 2011 Jan 13
[PubMed PMID: 21236695]
[28]
David TE, El Khoury G. Future directions on aortic valve-sparing operations. Annals of cardiothoracic surgery. 2023 Jul 31:12(4):366-368. doi: 10.21037/acs-2023-avs2-17. Epub 2023 Jul 19
[PubMed PMID: 37554708]
Level 3 (low-level) evidence
[29]
Price J, De Kerchove L, Glineur D, Vanoverschelde JL, Noirhomme P, El Khoury G. Risk of valve-related events after aortic valve repair. The Annals of thoracic surgery. 2013 Feb:95(2):606-12; discussion 613. doi: 10.1016/j.athoracsur.2012.07.016. Epub 2012 Sep 7
[PubMed PMID: 22959573]
[30]
Aicher D, Langer F, Lausberg H, Bierbach B, Schäfers HJ. Aortic root remodeling: ten-year experience with 274 patients. The Journal of thoracic and cardiovascular surgery. 2007 Oct:134(4):909-15
[PubMed PMID: 17903506]
[31]
Zuo Y, Tan R, Qin C. Outcomes of valve-sparing aortic root replacement in patients with bicuspid aortic valve and tricuspid aortic valve: a systematic review and meta-analysis. Journal of cardiothoracic surgery. 2023 Jul 3:18(1):206. doi: 10.1186/s13019-023-02329-8. Epub 2023 Jul 3
[PubMed PMID: 37400892]
Level 1 (high-level) evidence
[32]
Boodhwani M, El Khoury G. Aortic valve repair: a glimpse into the future. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012 Jan:41(1):2-3. doi: 10.1093/ejcts/ezr013. Epub
[PubMed PMID: 22219496]
[33]
de Heer F, Lansac E, El-Hamamsy I, Pibarot P, De Kerchove L, El Khoury G, Schäfers HJ, Takkenberg JJM, Kluin J. The AVIATOR registry: the importance of evaluating long-term patient outcomes. Annals of cardiothoracic surgery. 2019 May:8(3):393-395. doi: 10.21037/acs.2019.04.08. Epub
[PubMed PMID: 31240184]
[34]
Baric D, Sliskovic N, Sestan G, Gjorgjievska S, Unic D, Kusurin M, Varvodic J, Safaric Oremus Z, Jurin I, Bulj N, Susnjar D, Rudez I. Aortic Valve Repair with External Annuloplasty in Bicuspid versus Tricuspid Aortic Valve Patients. Journal of cardiovascular development and disease. 2024 Jan 6:11(1):. doi: 10.3390/jcdd11010017. Epub 2024 Jan 6
[PubMed PMID: 38248887]
[35]
Ram D, Bouhout I, Karliova I, Schneider U, El-Hamamsy I, Schäfers HJ. Concepts of Bicuspid Aortic Valve Repair: A Review. The Annals of thoracic surgery. 2020 Apr:109(4):999-1006. doi: 10.1016/j.athoracsur.2019.09.019. Epub 2019 Oct 11
[PubMed PMID: 31610163]