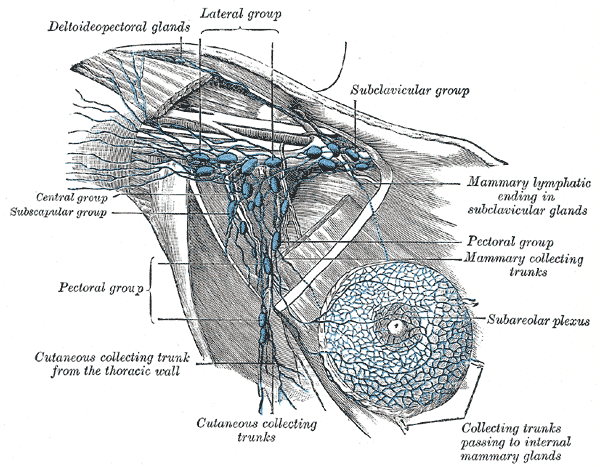
[1]
Black DM, Mittendorf EA. Landmark trials affecting the surgical management of invasive breast cancer. The Surgical clinics of North America. 2013 Apr:93(2):501-18. doi: 10.1016/j.suc.2012.12.007. Epub 2013 Jan 29
[PubMed PMID: 23464699]
[2]
Ling DC, Iarrobino NA, Champ CE, Soran A, Beriwal S. Regional Recurrence Rates With or Without Complete Axillary Dissection for Breast Cancer Patients with Node-Positive Disease on Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy. Advances in radiation oncology. 2020 Mar-Apr:5(2):163-170. doi: 10.1016/j.adro.2019.09.006. Epub 2019 Sep 27
[PubMed PMID: 32280815]
Level 3 (low-level) evidence
[3]
Wu D, Liu SY, Amina M, Fan ZM. [Normalization in axillary lymph node management after neoadjuvant therapy for breast cancer]. Zhonghua wai ke za zhi [Chinese journal of surgery]. 2019 Feb 1:57(2):97-101. doi: 10.3760/cma.j.issn.0529-5815.2019.02.005. Epub
[PubMed PMID: 30704211]
[4]
de Barros ACSD, de Andrade DA. Extended Sentinel Node Biopsy in Breast Cancer Patients who Achieve Complete Nodal Response with Neoadjuvant Chemotherapy. European journal of breast health. 2020 Apr:16(2):99-105. doi: 10.5152/ejbh.2020.4730. Epub 2020 Apr 1
[PubMed PMID: 32285030]
[5]
Costaz H, Rouffiac M, Boulle D, Arnould L, Beltjens F, Desmoulins I, Peignaux K, Ladoire S, Vincent L, Jankowski C, Coutant C. [Strategies in case of metastatic sentinel lymph node in breast cancer]. Bulletin du cancer. 2020 Jun:107(6):672-685. doi: 10.1016/j.bulcan.2019.09.005. Epub 2019 Nov 4
[PubMed PMID: 31699399]
Level 3 (low-level) evidence
[6]
Jung J, Kim BH, Kim J, Oh S, Kim SJ, Lim CS, Choi IS, Hwang KT. Validating the ACOSOG Z0011 Trial Result: A Population-Based Study Using the SEER Database. Cancers. 2020 Apr 11:12(4):. doi: 10.3390/cancers12040950. Epub 2020 Apr 11
[PubMed PMID: 32290437]
[7]
Cipolla C, Valerio MR, Grassi N, Calamia S, Latteri S, Latteri M, Graceffa G, Vieni S. Axillary Nodal Burden in Breast Cancer Patients With Pre-operative Fine Needle Aspiration-proven Positive Lymph Nodes Compared to Those With Positive Sentinel Nodes. In vivo (Athens, Greece). 2020 Mar-Apr:34(2):729-734. doi: 10.21873/invivo.11831. Epub
[PubMed PMID: 32111777]
[8]
Dixon JM, Cartlidge CWJ. Twenty-five years of change in the management of the axilla in breast cancer. The breast journal. 2020 Jan:26(1):22-26. doi: 10.1111/tbj.13720. Epub 2019 Dec 19
[PubMed PMID: 31854498]
[9]
Alvarado MD, Mittendorf EA, Teshome M, Thompson AM, Bold RJ, Gittleman MA, Beitsch PD, Blair SL, Kivilaid K, Harmer QJ, Hunt KK. SentimagIC: A Non-inferiority Trial Comparing Superparamagnetic Iron Oxide Versus Technetium-99m and Blue Dye in the Detection of Axillary Sentinel Nodes in Patients with Early-Stage Breast Cancer. Annals of surgical oncology. 2019 Oct:26(11):3510-3516. doi: 10.1245/s10434-019-07577-4. Epub 2019 Jul 11
[PubMed PMID: 31297674]
[10]
Ung O, Tan M, Chua B, Barraclough B. Complete axillary dissection: a technique that still has relevance in contemporary management of breast cancer. ANZ journal of surgery. 2006 Jun:76(6):518-21
[PubMed PMID: 16768781]
[11]
Gupta S, Gupta N, Kadayaprath G, Neha S. Use of Sentinel Lymph Node Biopsy and Early Physiotherapy to Reduce Incidence of Lymphedema After Breast Cancer Surgery: an Institutional Experience. Indian journal of surgical oncology. 2020 Mar:11(1):15-18. doi: 10.1007/s13193-019-01030-4. Epub 2020 Jan 7
[PubMed PMID: 32205962]
[12]
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, Ashikaga T, Weaver DL, Mamounas EP, Jalovec LM, Frazier TG, Noyes RD, Robidoux A, Scarth HM, Wolmark N. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. The Lancet. Oncology. 2010 Oct:11(10):927-33. doi: 10.1016/S1470-2045(10)70207-2. Epub
[PubMed PMID: 20863759]
Level 1 (high-level) evidence