Continuing Education Activity
The bidirectional Glenn (BDG) and hemi-Fontan are the types of surgical techniques used to create superior cavopulmonary anastomosis, the second stage repair in Fontan completion. This activity reviews pertinent anatomy and highlights the role of the interprofessional team in managing patients who undergo the bidirectional Glenn procedure.
Objectives:
Identify the indications of the bidirectional Glenn procedure.
Apply the bidirectional Glenn procedure technique.
Identify the potential complications of the bidirectional Glenn procedure.
Introduction
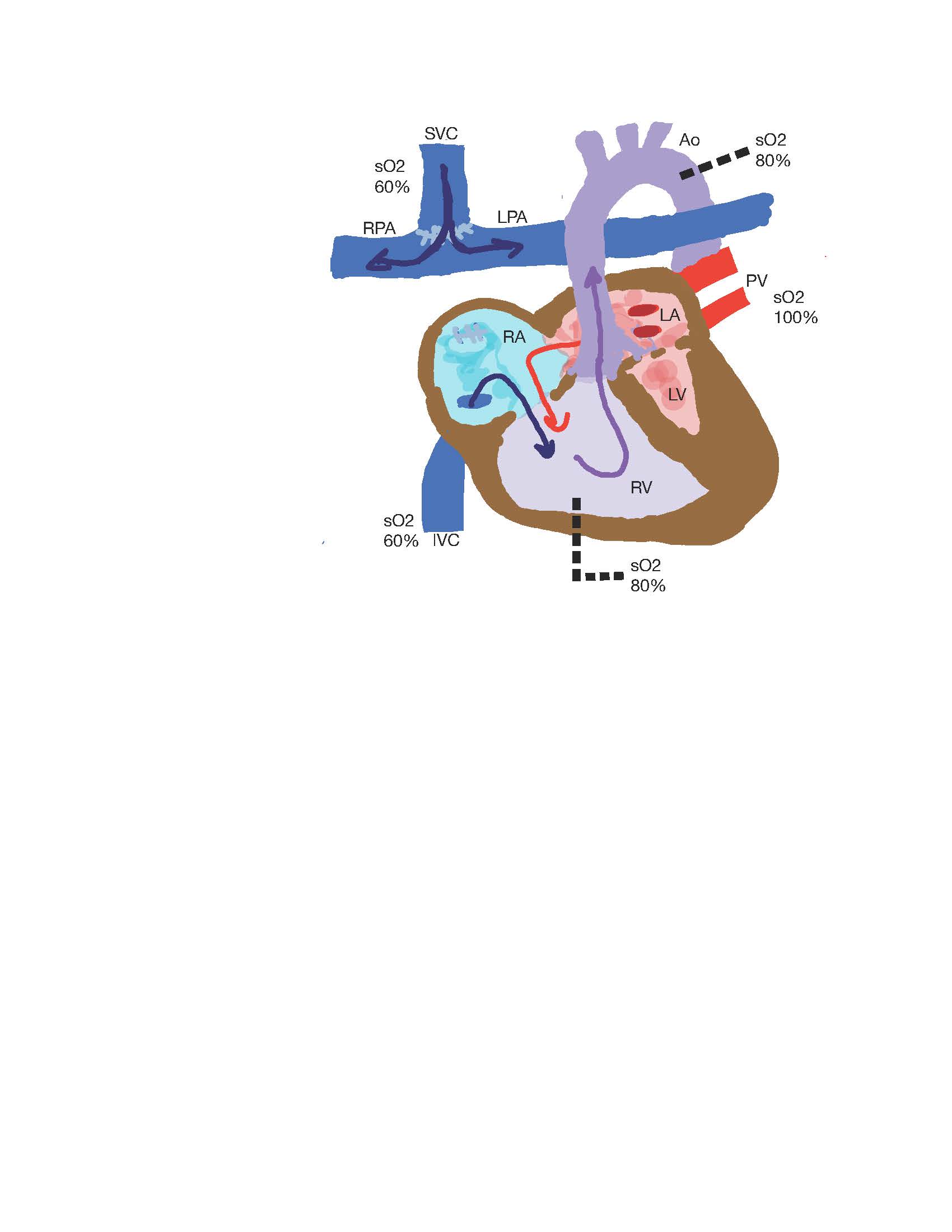
The bidirectional Glenn (BDG) and hemi-Fontan are surgical techniques used to create superior cavopulmonary anastomosis, the second stage repair in Fontan completion (see Image. Superior Cavopulmonary Anastomosis). They are performed in patients with an anatomic or functional single ventricle. Whether it is right or left, the single ventricle must, therefore, provide blood supply to the higher resistance systemic circulation and the lower resistance pulmonary circulation until surgical correction is undertaken. The BDG procedure, or hemi-Fontan, helps eliminate volume load on the single ventricle and simplifies the operative procedure at Fontan.
Several centers have reported decreased mortality and morbidity of the Fontan procedure in those who have undergone BDG or hemi-Fontan. All patients with a single ventricle must eventually undergo a Fontan procedure to achieve separation of their systemic and pulmonary circulations. A patient with a completed Fontan procedure has systemic venous return diverted to the pulmonary circulation, driven by central venous pressure and affected by intrathoracic pressure changes and systemic ventricular relaxation. As of 2018, there are an estimated 50,000 to 70,000 patients with a Fontan circulation, 40% of these patients older than 18.[1] About 1000 Fontan procedures are performed annually in the United States.[2]
Anatomy and Physiology
Patients requiring a BDG have circulation with complete parallel mixing of the systemic and pulmonary vascular beds. The BDG is undertaken to convert this circulation into a series circuit where pulmonary blood flow (PBF) is based upon systemic venous return. For optimal functioning of this circuit, blood flow through the pulmonary circulation cannot be inhibited by elevated pulmonary vascular resistance (PVR), AV valve dysfunction, or reduced ventricular compliance. In patients with HLHS, no obstruction to blood flow should exist at the atrial septum.
The BDG, or superior cavopulmonary anastomosis (SCPA), is undertaken to enable ventricular volume unloading associated with reduced ventricular end-diastolic volume and ventricular hypertrophy.[3] The earlier this repair is undertaken, the more likely these changes occur in a patient’s cardiac physiology. The BDG commonly occurs following the first-stage palliation, including a Blalock-Taussig (BT) shunt or pulmonary artery band. At the time of the BDG, the surgeon must decide whether to create or eliminate an additional source of pulmonary blood flow through a modified BT shunt or a PA band. Although the shunt improves arterial saturation, deoxygenated blood from the inferior vena cava (IVC) continues to mix with the pulmonary venous return at the atrial level. Before Fontan's completion, anatomic challenges should be addressed, including a restricted atrial septum, pulmonary artery stenosis, systemic outflow tract obstruction, atrioventricular valve regurgitation, and collateral vessels.
Norwood et al suggested that a superior cavopulmonary anastomosis or Hemi-Fontan procedure should be performed as a primary step before conversion to complete Fontan physiology. This would reduce volume overload on the single ventricle and avoid acute ventricular volume associated with a Fontan. With a Fontan, although ventricular cavity size is reduced, ventricular hypertrophy would result due to the persistence of ventricular mass. In effect, reduced ventricular compliance would lead to Fontan circulation failure. With the BDG, ventricular remodeling would occur slowly over time with the tandem reduction in ventricular mass and wall thickness. The BDG was also postulated to improve systemic arterial oxygen saturation without an increase in ventricular workload or elevated PVR.[3]
Indications
Common indications for a bidirectional Glenn or hemi-Fontan include hypoplastic left heart syndrome (HLHS), tricuspid atresia, double inlet left or right ventricle, pulmonary atresia with an intact ventricular septum, unbalanced atrioventricular canal defects, congenitally corrected transposition of the great arteries, and anatomic variants that cause significant hypoplasia of either ventricle. Based on analyses from the Single Ventricle Reconstruction Trial public data set, the bidirectional Glenn (BDG) procedure has its lowest mortality risk if undertaken 3 to 6 months following Stage I repair or the Norwood procedure.[4]
Of the 399 patients who survived a BDG, the 3-year survival rate was 87%, and the overall transplant-free survival was 68%. Analysis from the National Pediatric Cardiology Quality Improvement Collaborative (NPC-QIC) registry revealed that patients with a BDG performed after 5.1 months of age had higher mortality, suggesting a marked benefit for earlier surgical correction.[5] Those infants who underwent a BDG before 4 months of age had increased mortality, likely due to coexisting disease and other high-risk clinical attributes.[6]
Contraindications
Moderate-to-severe pulmonary hypertension is considered to be a relative contraindication to the performance of the BDG. It requires the creation of bilateral bidirectional Glenn shunts if there is a persistent left superior vena cava.
Equipment
Transesophageal echocardiography (TEE) imaging is utilized to identify the superior vena cava (SVC) and the anastomoses to the right pulmonary artery (PA). Pulsed Doppler and color-flow imaging evaluate for a thrombus or stenosis at the anastomosis site. Pulmonary arteriovenous fistula is excluded by injecting agitated saline solution peripherally. Expected findings at the shunt include low-velocity, laminar, or biphasic flow with an inspiratory increase in flow velocity during spontaneous ventilation. Turbulent flow at the level of the anastomosis may indicate an obstruction.[7] Extracorporeal membrane oxygenation (ECMO) might be needed briefly in the post-operative period if there is cardiac dysfunction or severe pulmonary hypertension.
Personnel
Preparation, procedure, and post-procedure of bidirectional Glenn shunt require a multidisciplinary approach. Before the procedure, the case must be discussed by cardiologists, interventional cardiologists, and cardiothoracic surgeons using echocardiography and cardiac catheterization data to determine if the patient is appropriate for BDG. The operation involves the whole team of operating nurses, cardiac anesthesiologists, and perfusionists.
Postprocedure, these patients need intensive care for a week and help from the intensivists, intensive care nurses, and respiratory therapists. Sometimes, these patients need Extracorporeal membrane oxygenation (ECMO) in the postoperative period and need an ECMO team ready to go.
Preparation
The BDG is not formed any earlier than 2 months of age because PVR is too high at this time to allow passive PBF and systemic oxygenation. Timing for an early BDG includes worsening cyanosis following a Stage I repair or residual aortic coarctation. However, these patients tend to have a complicated postoperative course secondary to elevated PVR. There are also select patients who do not require stage I palliation, and the BDG is their first operative intervention.
Before surgical repair, patients should undergo cardiac catheterization or cardiac MRI to ascertain anatomy and physiology. Before surgical intervention, the surgeon should know the patient’s PVR, ventricular end-diastolic pressure, AV valve function, and residual blood flow obstruction at the atrial septum remnant. Anatomic variants must be investigated, primarily alternative venous communications between sinus venous drainage and the myocardium or IVC. These vessels can result in postoperative hypoxemia as venous return from the upper body bypasses the pulmonary circulation. A thorough preoperative evaluation should elucidate multisystemic organ function. Patients treated with anticoagulants or platelet inhibitors should have these medications discontinued before surgery. Many institutions will discontinue aspirin for 7 to 10 days preoperatively, although it is useful in maintaining BT shunt patency.[8]
Technique or Treatment
The BDG is done with support from most institutions' cardiopulmonary bypass (CPB). CPB is instituted with cannulae in the aorta, superior vena cava (SVC), and the right atrium (RA). During repair, mild hypothermia is utilized (32-34 ° C), and aortic cross-clamping is avoided for a primary BDG without additional intracardiac procedures. Following the institution of CPB, the BT shunt, or primary right ventricle (RV) to pulmonary artery (PA) conduit, is occluded and promptly divided and oversewn. The common surgical technique includes transection of the SVC from its RA junction, with the integrity of the sinoatrial (SA) node maintained. The RA is overseen, and the SVC is anastomosed to the right PA. Inadequate SVC size or suboptimal flow characteristics in the central branch PAs can lead to compromised Glenn shunt flow.
Often, patients have some degree of pulmonary atresia, either congenitally or secondary, due to the incorporation of the pulmonary valve into neonatal palliation during stage 1 repair. The pulmonary valve leaflets are sewn together to avoid pulmonary valve sinus thrombus formation, which can cause a shower of retrograde emboli into the single systemic ventricle. In patients with tricuspid atresia, RV hypoplasia, or Ebstein’s anomaly, a "pulsatile Glenn" shunt refers to some ejection of blood from the pulmonary ventricle through the PA, which is instrumental in elevating PA pressure and producing a higher pulse pressure. This configuration is utilized in patients where the RV is too hypoplastic to receive all SVC and inferior vena cava (IVC) blood flow. During surgery, a clinical decision must be made regarding maintaining or eliminating additional PBF (APBF) via a BT shunt or PA band. Multiple analyses have shown that APBF is associated with higher PA pressure, improved oxygenation, and an increased incidence of chylothoraces and pleural effusions.[7]
During BDG repair, additional surgical interventions, including PA augmentation, correction of residual coarctation following Stage I repair, atrial septectomy, AV valve annuloplasty, or regurgitation repair, may be appropriate. For patients with HLHS, aortic reconstruction and atrial septectomy often accompany the BDG procedure. Patients with heterotaxy syndrome add another layer of complexity to surgical correction. These patients often have bilateral SVCs, and the left SVC is anastomosed to the left PA. At the time of the BDG, heterotaxy patients also require revision of anomalous pulmonary venous drainage, necessitating aortic cross-clamp placement. In heterotaxy patients with an interrupted IVC, the suprahepatic IVC is absent, and systemic blood below the diaphragm returns via collateral circulation via the azygos or hemiazygous circulation. The SCPA will then divert all caval blood flow to the BDG circuit, known as the Kawashima procedure.[9] These patients will eventually require a completion Fontan, or they tend to develop pulmonary arteriovenous fistulae with profound hypoxia secondary to right-to-left intrapulmonary shunting.
Some surgeons perform the hemi-Fontan procedure instead of the standard BDG, which involves anastomosis of the SVC to the PA and the RA to the PA confluence. Anastomosis to the left PA is augmented with a homograft patch, which creates direct blood flow from the upper RA to the PAs. This procedure may cause SA node injury, although it may make the final stage of Fontan completion more straightforward. An isolated BDG can also be performed via a shunt from the SVC to the RA with partial heparinization. Surgical intervention without CPB is undertaken to avoid hypoxic-ischemic brain injury during occlusion of the SVC.
Classically, BDG is performed with the aid of CPB to ensure a pristine surgical field and uniform anastomosis, as well as the avoidance of neurologic injury during SVC clamping. During SVC clamping, cerebral perfusion parameters, such as near-infrared spectroscopy, SVC pressure, and systemic pressure, should be monitored. Off-pump BDG has been successfully utilized for isolated BDG. However, risks include decreased cerebral oxygenation, global electroencephalographic slowing, and up to a 50% reduction in middle cerebral artery blood flow velocity during SVC clamping.[10] SVC decompression techniques to combat these complications, including SVC to right atrial shunting, SVC to contralateral PA shunt, and maintaining azygos vein blood flow during SVC clamping, if possible.[11]
Intraoperatively, all standard non-invasive monitors and an intra-arterial catheter are placed following tracheal intubation. Cannulation of the jugular or subclavian veins is avoided due to thrombosis concerns. The femoral vein is usually cannulated for central venous access, while a small catheter is placed in the internal jugular vein to monitor PA pressure for 12-24 hours postoperatively. Most patients exhibit favorable hemodynamics as PBF results from venous diversion from the upper body. A vasopressor may be necessary in some instances, but not always. Infants with some degree of diastolic dysfunction receive an inodilator, such as milrinone.
Intraoperative TEE assesses volume status, ventricular contractility, atrioventricular valve integrity, and anastomotic patency. CVP should be maintained at 15 to 20 mmHg to enable passive pulmonary perfusion and maintain cardiac output. The transpulmonary gradient, the difference between the CVP and left atrial pressure, determines the pulmonary blood flow. The normal transpulmonary gradient should be between 5 and 8 mm Hg, and an elevated transpulmonary gradient indicates high pulmonary vascular resistance (PVR) and needs to be addressed by avoiding hypoxia/acidosis or using nitric oxide. A shortened inspiratory and prolonged expiratory time should be maintained when the patient is mechanically ventilated. Hemodynamic goals in these patients include the maintenance of inotropy but the reduction of PVR and SVR, which augments preload and avoids a reduction in cardiac output. The patient may require cardiac pacing postoperatively; sinus rhythm also maintains preload and cardiac output. Although extubation is preferred, this should be weighed against premature extubation, leading to hypercarbia, hypoxia, and acidosis, which can lead to worsening PVR.
Postoperatively, PVR should be minimized to allow forward passive flow through the pulmonary circuit. Before termination from CPB, the patient’s endotracheal tube should be carefully suctioned and cleared of secretions. Doppler flow studies have shown that PBF occurs during the expiratory phase of positive pressure ventilation following a BDG. Thus, respiratory rate and inspiratory time should be limited to 1 second.[12] A positive end-expiratory pressure (PEEP) greater than 6mmHg is avoided in these patients as it can significantly reduce cardiac index with elevated PVR.[13] Maintaining a higher PaCO (45-50 mmHg) can increase cerebral blood flow, augment PBF, and increase systemic oxygen delivery.[14] Nitric oxide can be utilized to lower PVR in patients with pulmonary hypertension. Modified ultrafiltration (MUF) has been shown to reduce postoperative blood loss and pleural and pericardial effusions in patients following a BDG.[15]
Complications
A traditional bidirectional Glenn procedure, undertaken to balance pulmonary and systemic blood flow via a shunt, has a mortality rate of less than 2 percent.[16] NPC-QIC data revealed that postoperative complications occurred in approximately 30% of patients and included emergency cardiac catheterization (9%), new onset of neurologic deficit (9%), reoperation (6%), cardiac arrest (3%), and feeding difficulties that necessitated fundoplication or gastrostomy tube placement (15%).[17] Multivariate analysis revealed that the most common risk factors for postoperative complications included the presence of mitral stenosis, aortic atresia, female gender, and failure to thrive perioperatively. In a smaller-sized patient, reduced SVC size can make cannulation challenging and lead to a gradient at the cannulation site after tying a purse-string suture. A large-bore venous cannula in the SVC in such a patient may impede cerebral venous drainage and lead to neurologic dysfunction.
Factors that exacerbate postoperative blood loss in this population include age less than 2 years, reoperation, use of aspirin, hypoxemia, and deep hypothermic bypass. Almost all patients undergoing a BDG will need a repeat sternotomy, with an increased risk for blood loss due to mediastinal adhesions and CPB-induced arrhythmias. Anesthesiologists and surgeons should discuss the need for antifibrinolytic agents, multiple large-bore intravenous access for administering blood products, and the application of external cardioversion or defibrillation pads. In addition, a CPB circuit should be primed, and heparin should be available in case of an emergent need for a bypass.
Following the completion of the BDG, exacerbated hypoxemia is one of the most common postoperative complications caused by a suboptimal ventilation strategy or diminished PBF. If oxygenation does not improve the following optimization of volume status and ventilation strategy, collateral vessels from the upper body to the systemic circulation should be investigated. These vessels may require coil embolization in a persistently hypoxemic patient. Early extubation is recommended in these patients as spontaneous ventilation will augment cardiopulmonary-cerebral circulation, and PaO2 and PaCO2 should be optimized. Elevating the head of the bed and maintaining the neck in a neutral position will enhance cerebrovenous drainage and prevent hypoxemia. Systemic hypertension can occur in patients following a BDG as the cerebral circulation requires a higher venous pressure following surgical intervention. Following the BDG, cerebral venous pressure is equal to PA pressure and elevated to a mean of 12 to 18 mmHg. Hypertension can result from the body's aim to maintain cerebral perfusion pressure.
Pleural effusions are one of the important complications postoperatively that can prolong the duration of hospitalization and are known to happen in 9% of children. Though the cause remains uncertain, increased left-to-right shunting from some collaterals can be the cause in some cases. Sometimes, pulmonary arteriovenous (AV) fistulas can be formed in children who have undergone the BDG procedure and Kawashima operation, as these exclude hepatic venous circulation to the lungs on the first pass, suggesting a hepatic factor as a cause for pulmonary AV fistulas, which can cause desaturations.
Patients with failing BDG physiology develop elevated venous pressures that reduce PBF, impair oxygenation, and inhibit cerebral venous drainage. Decreased cerebral perfusion can result from high cerebral venous pressures in low systemic blood pressure. The failing Glenn circuit is often caused by atrioventricular valve regurgitation, leading to reduced cardiac output. Jolley et al identified seizures, cerebral hemorrhage, and embolic stroke as common complications in this population. If a BDG patient requires extracorporeal membrane oxygenation (ECMO) support, increased morbidity and mortality are associated with renal injury, neurologic injury, and persistent acidosis.[18][19] Analysis from the NPC-QIC revealed that the use of ECMO does not predict prolonged hospital stay following BDG.[20]
Clinical Significance
The development of the BDG procedure has been a disjointed and arduous process. Right heart bypass procedures were first instituted to manage cyanotic infants with tricuspid atresia. Although up to 40 techniques have been described in the literature, a few noteworthy ones are worth mentioning. Rodbard and Wagner first described right ventricular bypass by connecting the right atrial appendage to the right PA while ligating the main PA in canines. Shortly thereafter, Carlon et al described the first superior cavopulmonary connection in a dog, utilizing a venous shunt to increase pulmonary blood flow instead of a BT shunt. This group ligated the SVC, creating an end-to-end anastomosis between the RPA and the azygos vein.[21][22]
Glenn’s group at Yale began experimenting with transection of the RPA with subsequent anastomosis of the SVC and or IVC to either the right or main PA.[23] The survival rate at 10 months for these animals was low (8.8%), and nearly one-third developed chylous effusions. This group also described the complications that can result from cavopulmonary connections, including pleural effusions, ascites, and thrombosis.[24] Alex Haller introduced the concept of the bidirectional superior cavopulmonary connection in 1964.[25] He first instituted the end-to-side SVC to RPA anastomosis in an experiment including 50 dogs. This connection provided bidirectional blood flow to both pulmonary beds while reducing SVC obstruction and creating a larger cavopulmonary anastomosis.[26] The first successful superior cavopulmonary bypass was performed by Glenn in 1958 on a 7-year-old male with a single ventricle and transposition of the great arteries.[27]
Circulation following the BDG is characterized by a volume-unloaded ventricle, passive pulmonary blood flow, and cyanosis due to inferior vena cava supply of blood to systemic arterial output. Oxygen saturation lingers in the mid-’80s in patients with a BDG. Cyanosis is exacerbated in these infants as they age during periods of exercise and exertion, including walking and crawling, at this point, Fontan completion should be considered.[28] Ventricular dysfunction in these patients is associated with increased mortality.
Enhancing Healthcare Team Outcomes
Experienced institutions have excellent outcomes following the BDG, with operative survival over 96%.[29] Infants with a stage 1 repair involving a Sano modification may require an earlier BDG repair at 3 months but have not shown increased morbidity or mortality.[30] There has been some push to encourage an earlier BDG repair to reduce interstage mortality.[31]
Jaquiss et al reported on an earlier BDG in 85 patients with HLHS. Patients were divided into surgical intervention at 4 months (33 patients) and greater than 4 months (52 patients) and compared for preoperative and postoperative variables. Six-year survival and functional assessment were not different between the 2 groups, although the group of younger patients exhibited more profound hypoxemia and required longer hospitalization. Ultimately, the younger group exhibited a similar outcome following Fontan's completion. Carlo et al investigated 92 patients 30 days following BDG repair; 8 of these patients died, and 3 required a cardiac transplant.[32] These complications were associated with severe tricuspid valve regurgitation and low weight at the time of BDG.[30]
Nursing, Allied Health, and Interprofessional Team Interventions
A good operating team, along with pediatric cardiac anesthesiologists, OR nurses, and perfusionists running the cardiopulmonary bypass, are required for the procedure to go smoothly. There should be a good handoff between the OR team and the ICU team that receives the patient after the surgery. An interprofessional ICU team of nurses trained in cardiac intensive care, cardiac intensivists, and respiratory therapists is vital in taking good care of the patients post-operatively.
Nursing, Allied Health, and Interprofessional Team Monitoring
Monitoring of these children at every step is important from the time of procedure until the child gets discharged from the hospital. The teamwork of the above-mentioned personnel helps to minimize prevent, and treat the complications that arise during hospitalization.