Introduction
Any physical or psychological stimuli that disrupt homeostasis result in a stress response. The stimuli are called stressors, and physiological and behavioral changes in response to exposure to stressors constitute the stress response. A stress response is mediated through a complex interplay of nervous, endocrine, and immune mechanisms, activating the sympathetic-adreno-medullar (SAM) axis, the hypothalamic-pituitary-adrenal (HPA) axis, and the immune system.[1] The stress response is adaptive to prepare the body to handle the challenges presented by an internal or external environmental challenge, such as stressors. For example, the body's physiological responses to trauma and invasive surgery serve to attenuate further tissue damage. Suppose the exposure to a stressor is actually or perceived as intense, repetitive (repeated acute stress), or prolonged (chronic stress). In that case, the stress response is maladaptive and detrimental to physiology. Exposure to chronic stressors can cause maladaptive reactions, including depression, anxiety, cognitive impairment, and heart disease.[2]
Not all forms of stress are detrimental. Some stressors are enjoyable, stimulating, and inspiring. Termed eustress, these positive stressors replenish our energy, enhance cardiovascular health, boost endurance, and sharpen cognitive function. Eustress fosters mental acuity and motivation. In contrast, distress is characterized by adverse effects on the body and mind.
Stress is categorized into various types based on duration, source, and response.
- Acute stress: The short-term stress that typically results from immediate stressors or challenging situations. The body's fight-or-flight response leads to temporary physiological changes such as increased heart rate and adrenaline release.
- Chronic stress: This occurs when the stressor persists over an extended period. Prolonged exposure to chronic stress can lead to cumulative physiological and psychological effects, increasing the risk of health problems such as cardiovascular disease, anxiety, and depression.
- Episodic acute stress: The stress occurs when individuals experience frequent episodes of acute stress. This pattern may be characteristic of individuals who lead chaotic or disorganized lifestyles, constantly facing deadlines, commitments, or interpersonal conflicts. The cycle of stress exacerbates health issues and impairs daily functioning.
- Traumatic stress: This type results from exposure to traumatic events, such as natural disasters, accidents, or violent acts. The trauma overwhelms an individual's ability to cope and may lead to symptoms of posttraumatic stress disorder (PTSD), including intrusive memories, avoidance behaviors, and hyperarousal.
- Environmental stress: This type arises from adverse or challenging conditions in one's surroundings, including noise, pollution, overcrowding, or unsafe living conditions. These stressors can have detrimental effects on physical and mental health, contributing to a sense of discomfort or unease.
- Psychological stress: The stress stems from cognitive or emotional factors, such as perceived threats, worries, or negative thoughts. Typical stressors include work-related pressures, academic expectations, social comparisons, or self-imposed demands. Manifestations include anxiety, rumination, or perfectionism.
- Physiological stress: Physiological stress refers to the body's response to internal or external stressors that disrupt homeostasis. Examples include illness, injury, sleep deprivation, or nutritional deficiencies, which activate physiological stress pathways and compromise health and well-being.[3][4][5][6]
Cellular Level
The physiology of stress response has 2 components—a slow response mediated by the HPA axis and a fast response mediated by the SAM axis.
Sympathetic-Adreno-Medullar System
The quick response triggered by SAM activation leads to increased secretion of norepinephrine and epinephrine from the adrenal medulla into the circulation and increased secretion of norepinephrine from the sympathetic nerves, resulting in elevated levels of norepinephrine in the brain.[7] The released epinephrine and norepinephrine interact with α- and β-adrenergic receptors in the central nervous system and on the cell membrane of smooth muscles and other organs throughout the body. When released, norepinephrine and epinephrine bind to specific membrane-bound G-protein receptors to initiate an intracellular cyclic adenosine monophosphate (cAMP) signaling pathway that rapidly activates cellular responses. The activation of these receptors results in the contraction of smooth and cardiac muscle cells, leading to vasoconstriction, increased blood pressure, heart rate, cardiac output, skeletal muscle blood flow, increased sodium retention, increased levels of glucose due to glycogenolysis and gluconeogenesis, lipolysis, increased oxygen consumption, and thermogenesis. The physiological response also reduces intestinal motility, cutaneous vasoconstriction, and bronchiolar dilatation. In addition, SAM activation causes behavioral activation, such as enhanced arousal, alertness, vigilance, cognition, focused attention, and analgesia.
Hypothalamic-Pituitary-Adrenal System
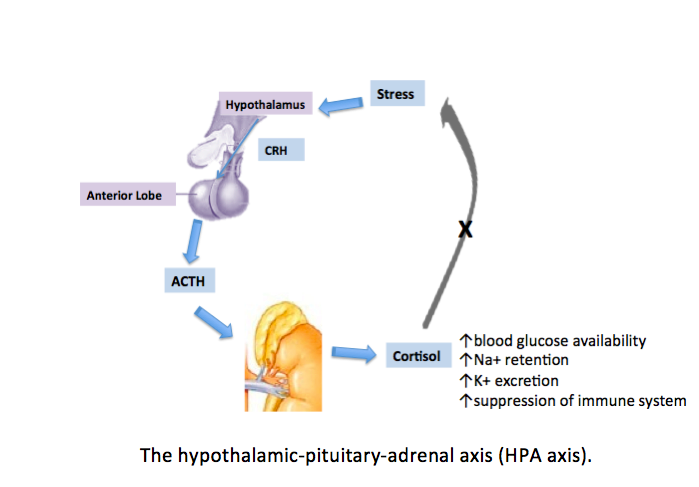
The slow response is due to the activation of the HPA axis, releasing corticotropin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus into the circulation. The CRH released from the hypothalamus acts on 2 receptors—CRH-R1 and CRH-R2. CRH-R1, widely distributed in the mammalian brain, is the key receptor for the stress-induced adrenocorticotropic hormone (ACTH) release from the anterior pituitary. CRH-R2 is expressed primarily in peripheral tissues, including skeletal muscles, gastrointestinal tract, and heart, and in subcortical structures of the brain. CRH-binding protein (CRH-BP) has a greater binding affinity with CRH compared to receptors. CRH-BP is expressed in the liver, pituitary gland, brain, and placenta.[8] The role of CRH-BP as a controller of the bioavailability of CRH is supported by studies finding that CRH-BP binds 40% to 60% of CRH in the brain.[9] In exposure to stress, the expression of CRH-BP increases in a time-dependent manner, a negative feedback mechanism to decrease the interaction of CRH with CRH-R1.[2] Serum cortisol level describes the body's total cortisol level, of which 80% is bound to cortisol-binding globulin and 10% is bound to albumin. Unbound cortisol is biologically active.
The released CRH then stimulates the anterior pituitary gland to release ACTH into the bloodstream. ACTH stimulates the adrenal cortex to secrete glucocorticoid hormones, such as cortisol, into the circulation. The inactive form of cortisol, cortisone, is catalyzed to the active form, cortisol, by 11-β-hydroxysteroid dehydrogenases (see Image. The Hypothalamic-Pituitary-Adrenal Axis).[10]
The HPA axis is regulated by pituitary adenylate cyclase-activating polypeptide. Pituitary adenylate cyclase-activating polypeptide may play a role in the production of CRH and modulate multiple levels of the HPA axis. Evidence also points to the involvement in the autonomic response to stress through increased secretion of catecholamines.[11] The pituitary adenylate cyclase-activating polypeptide receptors are G-protein coupled, and the R1 receptor is the most abundant in both central and peripheral tissues. This polypeptide may also modulate estrogen's role in potentiating the acute stress response.[12]
After CRH is released, the hormone binds with CRH-BP because CRH has a higher affinity with CRH-BP compared to the receptors. CRH-BP is expressed in the liver, pituitary gland, brain, and placenta.[5] The role of CRH-BP as a controller of the bioavailability of CRH is supported by studies finding that CRH-BP binds 40% to 60% of CRH in the brain.[6] In exposure to stress, the expression of CRH-BP increases in a time-dependent manner, which is believed to be a negative feedback mechanism that decreases the interaction of CRH with CRH-R1.[2] Serum cortisol level describes the body's total cortisol level, of which 80% is bound to cortisol-binding globulin and 10% is bound to albumin. Unbound cortisol is biologically active.
Organ Systems Involved
Stress generally affects all body systems, including cardiovascular, respiratory, endocrine, gastrointestinal, nervous, muscular, and reproductive systems. The endocrine system increases the production of steroid hormones, including cortisol, to activate the body's stress response. In the nervous system, stress triggers the sympathetic nervous system, prompting the adrenal glands to release catecholamines. Once the acute stress-induced crisis subsides, the parasympathetic nervous system aids in the body's recovery.
Cardiovascular System
Acute stress causes an increase in heart rate, stronger heart muscle contractions, dilation of the heart, and redirection of blood to large muscles. In contrast, chronic stress induces sustained activation of the sympathetic nervous system and HPA axis, leading to elevated levels of stress hormones such as cortisol and epinephrine.[13] The presence of these stress hormones promotes oxidative stress, endothelial dysfunction, and inflammation, thereby promoting the development of atherosclerosis and compromising vascular function. Moreover, stress-related alterations in lipid metabolism also contribute to dyslipidemia, exacerbating cardiovascular risk.[14]
Respiratory System
The respiratory and cardiovascular systems play crucial roles in supplying oxygen to the body's cells and eliminating carbon dioxide waste. Acute or chronic stress triggers dysregulation of the autonomic nervous system. This dysregulation can lead to a cascade of physiological effects, inducing bronchial hyperresponsiveness and inflammation. More importantly, acute stress can result in changes in breathing patterns due to airway constriction, leading to shortness of breath and rapid shallow breathing, exacerbating respiratory symptoms. Chronic stress also compromises immune function, increasing susceptibility to respiratory infections and exacerbating conditions such as asthma and chronic obstructive pulmonary disease. Furthermore, stress-induced alterations in inflammatory cytokines contribute to airway inflammation and mucus production.
Gastrointestinal System
Catecholamines, such as epinephrine and norepinephrine, released during stress profoundly affect the gastrointestinal system. These hormones bind to adrenergic receptors distributed throughout the gastrointestinal tract, influencing various physiological processes. By activating α-adrenergic receptors in the smooth muscle of the intestines, they cause delayed gastric emptying and reduced intestinal transit (motility).[15] Vasoconstriction in the gastrointestinal vasculature through α-adrenergic receptor activation reduces blood flow to the gut, thus inhibiting gastrointestinal secretions and nutrient absorption.[16]
Stress-induced changes in gut motility can manifest as diarrhea or constipation, whereas increased visceral sensitivity may contribute to symptoms such as irritable bowel syndrome. In addition, stress impairs the integrity of the gastrointestinal mucosal barrier, leading to increased permeability and susceptibility to inflammation and infection. Dysregulation of the gut-brain axis, mediated by stress, exacerbates gastrointestinal disorders and can worsen symptoms through bidirectional communication between the central nervous system and the gut microbiota.[17] Furthermore, stress-related alterations in gut microbiota composition and diversity can further contribute to gastrointestinal dysfunction, highlighting the intricate interplay between stress and gastrointestinal health.
Musculoskeletal System
Chronic stress elicits a cascade of physiological responses, including increased secretion of stress hormones such as cortisol and catecholamines, which impact the musculoskeletal system. Prolonged exposure to elevated levels of cortisol can lead to muscle wasting and decreased bone density by inhibiting osteoblast activity and promoting osteoclast function. In addition, activation of the stress-induced sympathetic nervous system can exacerbate musculoskeletal tension and contribute to conditions such as tension headaches, temporomandibular joint disorders, prolonged recovery from musculoskeletal injuries, and risk of developing conditions, including fibromyalgia and low back pain.
Immune System
When exposure to stress is chronic, the sympathetic nervous system, including the HPA axis, is activated, which can suppress innate and adaptive immune responses.[18] Prolonged elevation of cortisol levels suppresses immune function by inhibiting the production of pro-inflammatory cytokines and reducing the activity of immune cells, particularly lymphocytes. This immunosuppressive effect can increase infection susceptibility, delay wound healing, and exacerbate inflammatory conditions. In addition, chronic stress promotes systemic inflammation through the upregulation of inflammatory mediators, contributing to the pathogenesis of autoimmune diseases and chronic inflammatory disorders.[19]
Reproductive System
Chronic stress can disrupt the delicate balance of the reproductive axis by suppressing the secretion of gonadotropin-releasing hormone from the hypothalamus, which subsequently reduces the release of luteinizing hormone and follicle-stimulating hormone from the pituitary gland. Consequently, this disruption impairs ovarian function in women and reduces testosterone production in men. Chronic stress can also lead to menstrual irregularities, anovulation, and infertility in women, and impaired sexual desire, erectile dysfunction, and decreased sperm quality in men. In addition, stress-induced alterations in sex hormone levels and impaired reproductive function may contribute to conditions such as polycystic ovary syndrome and male hypogonadism.[20]
Function
The heightened autonomic response causes an increase in heart rate and blood pressure. During critical illness, the release of catecholamine decreases blood circulation to the gastrointestinal tract. During stress, plasma levels of norepinephrine and epinephrine redistribute blood volume to conserve the brain's blood supply. Stimulation of the sympathetic nervous system is varied but includes threats to the body such as hypoglycemia, hemorrhagic shock, exercise beyond the anaerobic threshold, and asphyxiation.[21] Epinephrine is also associated with active escape, attack, and fear.
A stressful situation, whether environmental or psychological, can activate a cascade of stress hormones that produce physiological changes. Activating the sympathetic nervous system in this manner triggers an acute stress response called the fight-or-flight response. This response enables an individual to either fight the threat or flee the situation. The rush of adrenaline and noradrenaline secreted from the adrenal medulla leads to a widespread discharge of almost all portions of the sympathetic system throughout the body. Physiological changes of this mass discharge effect include increased arterial pressure, more blood flow to active muscles, less blood flow to organs not needed for rapid motor activity, increased rate of blood coagulation, increased rates of cellular metabolism through the body, increased muscle strength, increased mental activity, increased blood glucose concentration, and increased glycolysis in the liver and muscle. The net effect of all these effects allows a person to perform more strenuous activity than usual. After the perceived threat disappears, the body returns to basal levels.
Mechanism
Physical stress stimulates the HPA and sympathetic nervous systems. Cortisol has various physiological effects, including catecholamine release, insulin suppression, mobilization of energy stores through gluconeogenesis and glycogenolysis, suppression of the immune-inflammatory response, and delayed wound healing.[22] B-cell apoptosis is an effect of the downregulation of the immune response.[23][24] Wound healing is also delayed due to the effects of collagen synthesis.[25] Aldosterone is a mineralocorticoid hormone that preserves blood pressure through sodium and water retention.
Glucocorticoid-binding receptors exist in the brain as mineralocorticoid and glucocorticoid receptors. The brain's first response to glucocorticoids is to preserve function. Glucocorticoid hormones such as cortisol, corticosterone, and dexamethasone have various effects of conserving energy and maintaining energy supply, such as reducing inflammation, restricting growth, producing power, and removing unnecessary or malfunctioning cellular components.[26]
Stress response is a nuanced interplay among diverse brain centers, particularly the neural mechanisms responsible for triggering stress reactions, which include the locus coeruleus, limbic system, and hypothalamic efferent activation complex.[7] These components are interconnected through various pathways, including ventral and dorsal adrenergic and serotonergic projections. The complex includes the locus coeruleus, hippocampus, septal-hippocampal-amygdaloid complexes, and anterior and posterior hypothalamic nuclei, serving as pivotal anatomical hubs for visceral and somatic efferent responses to emotional stimuli.[27] Essentially, the amygdala, particularly the central nucleus, plays a crucial role in processing emotional aspects of stress and initiating fear responses. The hippocampus, critical for memory formation, regulates the stress response by providing negative feedback to the hypothalamus, thus modulating cortisol release. The prefrontal cortex, involved in executive functions, including decision-making and impulse control, regulates stress responses through top-down inhibition of the amygdala and hypothalamus.[28] The dysregulation of these brain centers is implicated in stress-related disorders such as anxiety, depression, and PTSD.
Related Testing
Various testing techniques are used to measure stress response in humans.
- Biological markers: Assessing stress hormones such as cortisol, epinephrine, and norepinephrine levels in the blood, saliva, and urine provides objective indicators of the physiological stress response. These markers reflect the activity of the HPA axis and the SAM system. Sympathetic responses are also measurable through microneurography. The microneurography technique involves the insertion of an electrode into a peripheral nerve to measure sympathetic activity in the skin and muscles of the upper or lower limbs.
- Heart rate variability: Heart rate variability analysis assesses the variation in the time interval between consecutive heartbeats, reflecting the balance between the sympathetic (fight-or-flight) and parasympathetic (relaxation) nervous systems. Decreased heart rate variability is associated with sympathetic dominance and increased stress levels, whereas higher heart rate variability is associated with stress resilience and improved cardiovascular health.
- Electroencephalography: This method measures brainwave activity and can accurately gauge stress response. The findings of a study conducted in 2020 suggest that alpha asymmetry, an imbalance in alpha brainwave activity between brain hemispheres, is a potential stress biomarker. Mental health clinicians use neurofeedback to measure brainwaves and provide positive feedback during treatment.
- Electrodermal activity: This is measured by skin conductance or galvanic skin response and reflects changes in sweat gland activity in response to sympathetic arousal. Increased electrodermal activity indicates heightened physiological arousal and stress.
- Blood pressure and heart rate: Monitoring changes in blood pressure and heart rate provides insights into cardiovascular responses to stress, including increased sympathetic activation and vasoconstriction.[29][30][31][32]
Pathophysiology
General Adaptation Syndrome
General adaptation syndrome provides a framework for understanding the physiological responses to stress and the potential consequences of chronic stress on health and well-being. The syndrome describes the different stress-induced physiological changes through 3 different stages, with the last 2 stages showing the pathological changes of extended stress.[33] This syndrome is divided into the alarm reaction stage, resistance stage, and exhaustion stage.
The alarm reaction stage refers to the initial symptoms of the body under acute stress and the fight-or-flight response. After the initial shock of the stressful event, the body begins to repair itself by lowering cortisol levels and normalizing the physiologic reactions such as blood pressure and heart rate. During this recovery phase, the body remains alert until the stressful event is no longer triggering. However, if the stressful event persists for extended periods, the body adapts to cope with higher stress levels.[34] The body continues to secrete stress hormones, which maintain the body's elevated physical response to stress. This mechanism induces the resistance stage and includes symptoms such as poor concentration, irritability, and frustration. If the stressful event persists, the body enters the exhaustion stage. Symptoms of this stage include burnout, fatigue, depression, anxiety, and reduced stress tolerance. As the stressful event persists, the body's immune system weakens due to the suppressive effects of stress hormones on immune system cells.
Systemic Effects of Stress
Although the restoration of homeostasis is the goal of the stress response, chronic stress leads to dysfunctional responses, resulting in heart disease, stomach ulcers, sleep dysregulation, and psychiatric disorders. The HPA axis may become suppressed or dysregulated in these maladaptive responses to stress. Stress causes the cardiovascular system to respond with elevated blood pressure and heart rate; chronic activation of this response is a significant cause of cardiovascular disease. Coronary artery disease, stroke, and hypertension occur at a greater incidence in individuals with stress-related psychological disorders. The release of catecholamines in the stress response can have maladaptive effects on the gastrointestinal tract through decreased local blood flow. Chronic stress weakens the immune system, increasing the probability of developing Helicobacter pylori gastric ulcers and bleeding.[35] Sleep quality and quantity affect cortisol response to acute stress. Self-reported high sleep quality showed a strong cortisol stress response, whereas relatively good sleep quality showed a significantly weaker cortisol response in men. Regardless of gender, a blunted cortisol response to stress was observed in individuals experiencing difficulty staying awake and maintaining enthusiasm.[36]
Furthermore, diseases affecting the adrenal system, such as Addison's disease, Cushing's syndrome, and pheochromocytoma, influence the body's stress mechanisms by releasing cortisol and epinephrine. Addison's disease is characterized by a lack of glucocorticoid and/or mineralocorticoid hormones.[37] Conversely, Cushing's syndrome is marked by hypercortisolism due to endogenous or exogenous causes.[38] Pheochromocytomas are catecholamine-secreting tumors of the adrenal glands.[39]
Clinical Significance
The physiological responses of the body to stress have significant implications in various clinical applications, such as managing healthy patients, patients with hypoadrenalism undergoing surgery, and understanding the relationship between lifestyle changes and the stress response. The physiological stress of surgery causes cortisol levels to rise in a positive correlation to the severity of the surgery. In patients undergoing major surgeries as defined by the POSSUM scale, cortisol levels return to baseline on postoperative days 1 to 5. Postoperative pain severity does not correlate with cortisol levels after cardiac surgery.[21] A study examining cortisol levels during minor, moderate, and major surgeries found that postoperative opiate analgesia does not influence the stress cortisol response. For patients with hypoadrenalism who require cortisol replacement during surgery, the varied level of cortisol secretion correlated to the stress of surgical operations has implications.
For patients with hypoadrenalism undergoing surgery, hydrocortisone injections are administered to mimic cortisol levels observed in individuals with normal adrenal function during surgery; this is believed to help patients with hypoadrenalism withstand the physiological stress of surgery. Recommendations for dosage and supplementation methods vary. European guidelines suggest 100 mg of hydrocortisone intramuscularly before anesthesia, regardless of surgery type. Endocrine Society recommendations suggest 100 mg of hydrocortisone intravenously followed by infusion, which is the basis of the severity of the surgery. Testing cortisol levels in surgeries of varying severity shows that peak cortisol correlates with surgical severity, but peak cortisol levels are lower than previously suggested.[22]
Patients in the intensive care unit are subject to physical and environmental stress, and efforts are made to investigate the link between cortisol levels and illness recovery and to facilitate stressors during the stay to study outcomes. Subjective patient perception of relaxation is heightened with sleep adjuncts such as earplugs, eye masks, and relaxing music. However, these interventions did not influence nocturnal melatonin or cortisol levels.[40]
Long-term exercise can help prevent cardiovascular disease by adapting baseline cardiac performance. Long-term moderate exercise helps relieve stress-induced cardiovascular response by changing baroreflex set points in the nucleus of the tractus solitarius, thereby regulating blood pressure control and blood volume homeostasis regulated by the paraventricular nucleus.