Introduction
Bariatric surgery for weight loss has become a common practice in the United States, with about 179,000 operations performed in 2013. The second most common bariatric procedure done today is the Roux-en-Y gastric bypass (RYGB). To be a practicing health professional in the modern era, one must understand the more common chronic complications that may result from altering the gastrointestinal (GI) tract and how to manage these complications.
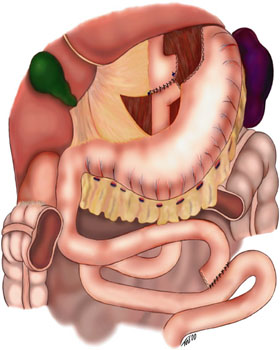
To understand the sequela of the operation, one must have a basic understanding of the anatomy of the GI tract and the resulting physiologic effects of altering that anatomy. Roux-en-Y gastric bypass involves creating a small gastric pouch (restricting food intake) connected to a roux limb (typically between 75 to 150 cm) which bypasses a large portion of the small intestine (preventing the absorption of nutrients.) This results in the food bolus bypassing most of the stomach (bypasses the part of the stomach containing most of the parietal cells and stomach acid), the duodenum, and the first 40 to 50 cm of jejunum. Nutrients will only be absorbed distal to these bypassed segments, and the majority will be absorbed in the "common channel," which is distal to where the biliopancreatic and the roux limb connect.
The common chronic complications following an RYGB are briefly described concerning epidemiology, presentation, diagnosis, and treatment.
Issues of Concern
Anastomotic Stricture
The most common location for stricture is the gastrojejunal anastomosis; this occurs between 3% to 7% following laparoscopic RYGB procedures (LRYGB). The pathophysiologies responsible for forming this stricture are ischemia, scarring, and inadequate technique (creating a small anastomosis). Patients will typically present weeks to months postoperatively with progressive dysphagia of solids to liquids and subsequent daily vomiting. Diagnosis is made with endoscopic evaluation. Treatment is primarily with endoscopic balloon dilation and in the setting of failure or multiple recurrences may need surgical revision.[1][2]
Marginal Ulceration
Marginal ulceration is described as an appearance of a peptic ulcer on the jejunal mucosa at the gastrojejunal anastomosis. It is diagnosed in 1% to 16% of patients who undergo LRYGB.[1] The pathology is a result of acid insult to the relatively unprotected jejunal mucosa. Several mechanisms have been implicated in marginal ulcers development:[3]
- Large pouch size (leading to a larger parietal cell mass in the pouch and resulting acid exposure)
- NSAIDs and smoking
- Mucosal ischemia
- Gastrogastric fistula (remnant stomach parietal cells producing acid which enters pouch leading to exposure of the jejunal mucosa to a higher acid level)
- Foreign body reaction (staples and sutures)
- Preoperative colonization of Helicobacter pylori
About one-third of patients will present within the first 3 months after surgery, and about one-half will present after 1 year. The most common presenting sign will be epigastric pain, and patients may less commonly present with nausea, vomiting, dysphagia, bleeding, or chronic anemia. Diagnosis is with upper endoscopy, and treatment is typically accomplished with proton pump inhibitors and repeat endoscopy to ensure healing. Medical treatment is successful in 85% to 95% of cases.[3] Complications of marginal ulceration include perforation, stricture, and bleeding. Perforated ulcers typically require emergent surgery with a graham patch closure and distal feeding tube placement. Strictures and bleeding unresponsive to medical and endoscopic measures may need a surgical revision of the gastrojejunostomy.
Gastro-Gastric Fistula (GGF)
GGF is an abnormal connection between the surgically created pouch and excluded remnant stomach. It occurs in approximately 1% to 2% of patients undergoing RYGB with a divided stomach (historically, the stomach was stapled and left undivided, leading to an unacceptable rate of GGF). Etiologies of a GGF include an incomplete transection of the stomach, anastomotic leak, marginal ulcer perforation, and foreign body erosion.[4] Patients will typically present with weight gain as the classic sign; however, intractable marginal ulceration may also GGF formation. Diagnosis is usually confirmed with a CT scan or UGI study. Treatment of GGF will include a PPI for all patients. Endoscopic treatments of small fistulae with clips may be successful; however, surgical treatment is usually necessary. Surgical options include remnant gastrectomy, transection of the fistulous transect, and revision of the gastrojejunal anastomosis.[5]
Cholelithiasis
Historically, the incidence of new gallstone formation following gastric bypass surgery ranges from 32% to 42%, and about one-third to one-half of those patients became symptomatic.[6] Increased gallstone formation in RYGB patients is caused by supersaturation of bile with cholesterol secondary to a reduction in bile acid secretion because of the caloric restriction. Another reason is limited gallbladder contractility and emptying secondary to a reduction in the secretion of cholecystokinin (CCK) due to the bypassed duodenum. Most gallstones are formed within the first 6 months postoperatively.
Management of gallstones with RYGB is a controversial subject with many options. First, we must understand that the addition of a cholecystectomy to a laparoscopic RYGB adds about 18 minutes and has added complications associated with it. All patients should be evaluated for gallstones either by preoperative ultrasound or intraoperative ultrasound. This will create 2 categories of patients based on the presence or absence of gallstones.
Presence of Gallstones
- Asymptomatic: Surgeon discretion to remove or leave the gallbladder
- Symptomatic: Most surgeons will elect to remove the gallbladder during RYGB surgery
Absence of Gallstones
Patients should receive ursodeoxycholic acid (UDCA) 600 mg per day for 6 months postoperatively, which effectively reduces gallstone formation to 2% within that timeframe. [7]
Choledocholithiasis
Patients may present with stones in the common bile duct and resultant cholangitis or pancreatitis. A useful diagnostic tool would be MRCP in this setting. The usual treatment, conventional ERCP, is not feasible since the duodenum is excluded and connected to the remnant stomach. Options in this situation are double-balloon enteroscopy, laparoscopic-assisted ERCP with a remnant stomach gastrostomy, percutaneous transhepatic cholangiography, and open common bile duct exploration.[8]
Small Bowel Obstruction (SBO)
In the setting of LRYGB, the incidence of SBO is between 1.5% to 5%.[9] The etiology of SBO in an RYGB patient can generally be attributed to internal hernias, adhesions, or strictures. There are three potential sites for internal hernia formation in an RYGB patient, depending on the technique used. If a retro colic roux limb technique is used, this creates a defect in the mesocolon, a defect in mesentery at jejunojejunostomy, and the Petersen’s defect (bordered by the roux limb mesentery, transverse mesocolon, and the retroperitoneum).[10] The number one cause of SBO in postoperative LRYGB has historically been an internal hernia. However, in patients who have undergone an ante-colic reconstruction, the most common cause may be a stricture or adhesions.[11] Historically when a patient had an open RYGB, there was a decrease in the formation of an internal hernia due to intraabdominal adhesions, which, in theory, does not allow the loops of the bowel to slip into the mesenteric defects.
Patients may present with vague, crampy abdominal pain with or without vomiting. This can be an acute or subacute presentation and may be intermittent. In a gastric bypass patient, these symptoms must be worked up promptly with a CT scan to look for an internal hernia. A CT scan has a lower sensitivity for diagnosing SBO in LRYGB when compared to the general public. Treatment is exploration, either laparoscopically or open, and specifics of the operation are related to the etiology of obstruction. In a patient with a negative CT scan but has continued obstructive symptoms, diagnostic laparoscopy must be considered.
Dumping Syndrome
Dumping syndrome can be classified as either early or late and encompass different clinical presentations. The prevalence of dumping syndrome post RYGB with a median follow-up of 4.5 years is about 13% and is more commonly found in young females.[12]
Early Dumping Syndrome
This occurs within the first hour after ingesting a meal (usually within 10 to 30 minutes) and is attributed to the rapid introduction of nutrients into the small bowel, causing an osmotically driven fluid movement into the small bowel lumen. This will typically present with diarrhea, dizziness, flushing, and possibly hypotension. First-line treatment is with a low carbohydrate, high protein/fiber diet taken in small/frequent meals. If one has persistent symptoms, then octreotide can be used. This is typically a self-limiting disease and subsides in 12 to 18 months following surgery.
Late Dumping Syndrome
This occurs between 1 to 3 hours after a meal and is a hypoglycemic response to hyperinsulinemia. This will present as tremors, diaphoresis, palpitations, and altered mental status. The first step in treatment is as above with diet modification and octreotide. If the effects of hypoglycemia are debilitating, then surgical intervention may be required. Accepted methods include bypass reversal to normal anatomy, surgeries to increase gastric reservoir, and conversion to sleeve gastrectomy.[13]
Nutritional Complications
B12
The incidence of low B12 one year postoperatively from an RYGB surgery is estimated to be around 30% to 35% but may be higher. Vitamin B12 is released from food by the action of stomach acid. An R binder protein then binds it. This bond is cleaved by pancreatic enzymes allowing B12 to complex with intrinsic factor(IF), a protein synthesized by parietal cells in the stomach. This complex is then absorbed in the terminal ileum. RYGB will alter all of the above processes leading to a vitamin B12 deficiency in the absence of supplementation. The liver can store a large amount of B12, which may defer the development of B12 deficiency syndrome for years. Patients are often asymptomatic but may present with megaloblastic anemia and neurological symptoms (paresthesias, unsteady gait, poor memory, agitation, confusion, depression.) Diagnosis is based on low serum vitamin B12 levels; if this is equivocal, it may be confirmed with methylmalonic acid and homocysteine levels. Treatment is primarily preventative as some of the sequelae are irreversible. Supplementation after surgery may be with daily orals or monthly injections.[14][15]
Folate
The incidence of low serum folate after RYGB is between 6% to 35%. Folic acid is absorbed through the majority of the jejunum, and deficiency usually results from an insufficient intake. Deficiency can result in megaloblastic anemia and sometimes with irritability, forgetfulness, or paranoid behavior. Treatment of deficiency is accomplished with a supplement of 1 mg per day, and symptoms will quickly resolve in 24 hours usually. Prevention is with 400 micrograms daily with a multivitamin.[16][17]
Thiamine
Thiamine deficiency is one of the most serious vitamin deficiencies after an RYGB. It is a water-soluble vitamin that is primarily absorbed in the jejunum. Only small amounts of thiamine are stored in the body, and a continuous exogenous supply is needed to maintain adequate levels. Deficiency may develop in the setting of decreased intake in an RYGB patient. A patient may present with a wide array of clinic syndromes which are well known as dry beriberi (neuropathic symptoms), wet beriberi (heart failure symptoms), or Wernicke’s encephalopathy (triad of ophthalmoplegia, ataxia, nystagmus). Patients with a subclinical deficiency may present with irritability, headaches, and fatigue. If symptomatic, the patient will need IV repletion and supplementation until all symptoms resolve. Prevention is with a daily multivitamin that contains 200% of the daily requirements.
Iron
Iron deficiency is the most common nutritional deficiency in an RYGB patient, with a 30% to 50% prevalence. Iron absorption is promoted when exposed to gastric acid and duodenal proteolytic enzymes resulting in molecular transformations. It can be absorbed at any level in the intestinal tract but is the most efficient in the duodenum. Deficiency in RYGB patients is due to the bypass of the mechanism mentioned above of absorption or slow chronic bleeding from marginal ulceration. Patients may present with fatigue and generalized weakness. Diagnosis is confirmed with laboratory tests showing microcytic anemia, elevated total iron-binding capacity, low ferritin, and low serum iron levels. Treatment is with oral supplementation, and if unsuccessful at correction or the patient is unable to tolerate the oral form, IV supplementation can be offered. Prevention is with daily oral supplementation, which may be included in a multivitamin. Menstruating women require higher doses than women who are not menstruating.
Calcium and Vitamin D
There is a high prevalence of vitamin-D deficiency and resulting secondary hyperparathyroidism in obese patients preoperatively. Obesity, however, results in a higher preoperative bone mineral mass. Calcium is preferentially absorbed in the duodenum and proximal jejunum, while vitamin D is primarily absorbed in the distal small bowel. After bypassing these segments, there is malabsorption of calcium with a resulting deficiency leading to a further increase in PTH. The long-term actions of PTH will release calcium from the bone causing osteopenia and eventually osteoporosis. Treatment and prevention are with oral supplementation.
Zinc, Copper, Selenium, Vitamin C
These can be deficient in RYGB patients and can rarely be of clinical significance. These are typically supplemented with a daily multivitamin.
Enhancing Healthcare Team Outcomes
The management of obesity requires an interprofessional team of providers, including an internist, primary care provider, nurse practitioner, dietitian, bariatric surgeon, sociologist, physical therapist, and an endocrinologist. The key to the prevention of obesity is patient education about changes in lifestyle and diet. Surgery is usually the last alternative, and while it does work, it is also expensive and associated with serious complications. Interprofessional care coordination includes working as a team during the preparation and performance of the procedure and also involves patient education and monitoring/follow-up. The interprofessional team approach will result in improved and more lasting patient outcomes. [Level 5]
Roux-en-Y gastric bypass is a commonly performed operation today in the United States. It has many advantages over other weight-loss surgeries but can present with early and late complications. Chronic complications include but are not limited to strictures, internal hernias, gastro-gastric fistulae, gallstones, marginal ulcers, dumping syndrome, and the nutritional deficiencies that accompany altering the GI tract. As increasingly more patients will have had this surgery, a healthcare practitioner must be aware of these sequelae and their management. Patients need to be made aware that surgery does not cure obesity- it is just a temporary method of inducing weight loss, and if the patient does not partake in exercise or a change in diet, the weight will soon be regained.