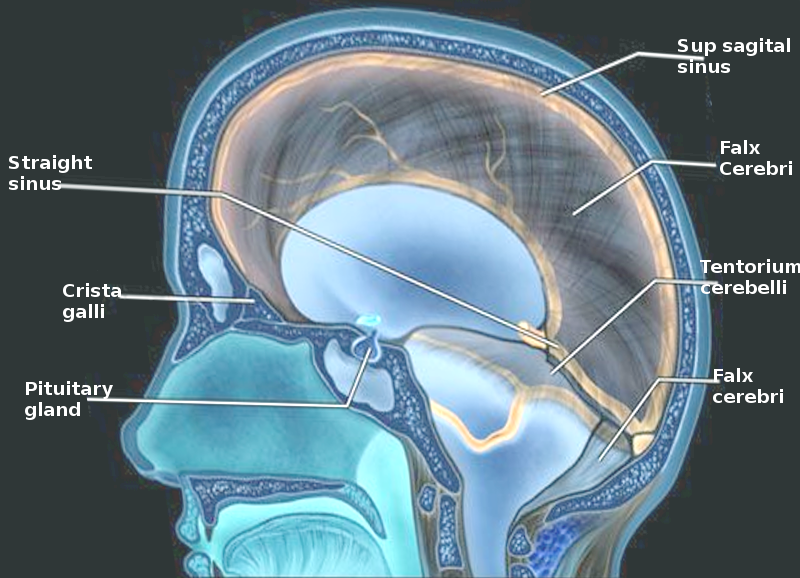
[1]
WELIKALA AH. Extensive calcification of the falx cerebri. The British journal of radiology. 1947 Jul:20(235):295
[PubMed PMID: 20249177]
[4]
O'Rahilly R, Müller F. The meninges in human development. Journal of neuropathology and experimental neurology. 1986 Sep:45(5):588-608
[PubMed PMID: 3746345]
[5]
Cayea PD, Balcar I, Alberti O Jr, Jones TB. Prenatal diagnosis of semilobar holoprosencephaly. AJR. American journal of roentgenology. 1984 Feb:142(2):401-2
[PubMed PMID: 6607617]
[6]
Pollock JA, Newton TH. The anterior falx artery: normal and pathologic anatomy. Radiology. 1968 Dec:91(6):1089-95
[PubMed PMID: 5699608]
[7]
Da Mesquita S, Fu Z, Kipnis J. The Meningeal Lymphatic System: A New Player in Neurophysiology. Neuron. 2018 Oct 24:100(2):375-388. doi: 10.1016/j.neuron.2018.09.022. Epub
[PubMed PMID: 30359603]
[8]
Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, Reich DS. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife. 2017 Oct 3:6():. doi: 10.7554/eLife.29738. Epub 2017 Oct 3
[PubMed PMID: 28971799]
[9]
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 Jul 16:523(7560):337-41. doi: 10.1038/nature14432. Epub 2015 Jun 1
[PubMed PMID: 26030524]
[10]
Lee SH, Shin KJ, Koh KS, Song WC. Visualization of the tentorial innervation of human dura mater. Journal of anatomy. 2017 Nov:231(5):683-689. doi: 10.1111/joa.12659. Epub 2017 Jul 10
[PubMed PMID: 28695607]
[11]
Lv X, Wu Z, Li Y. Innervation of the cerebral dura mater. The neuroradiology journal. 2014 Jun:27(3):293-8. doi: 10.15274/NRJ-2014-10052. Epub 2014 Jun 17
[PubMed PMID: 24976196]
[12]
Ryu CW. Persistent falcine sinus: is it really rare? AJNR. American journal of neuroradiology. 2010 Feb:31(2):367-9. doi: 10.3174/ajnr.A1794. Epub 2009 Sep 24
[PubMed PMID: 19779000]
[13]
Chaddad-Neto F, Devanir Silva da Costa M, Bozkurt B, Leonardo Doria-Netto H, de Araujo Paz D, da Silva Centeno R, Grande AW, Cavalheiro S, Yağmurlu K, Spetzler RF, Preul MC. Contralateral anterior interhemispheric-transcallosal-transrostral approach to the subcallosal region: a novel surgical technique. Journal of neurosurgery. 2018 Aug:129(2):508-514. doi: 10.3171/2017.4.JNS16951. Epub 2017 Nov 3
[PubMed PMID: 29099298]
[14]
Shaikh N, Dixit K, Raizer J. Recent advances in managing/understanding meningioma. F1000Research. 2018:7():. pii: F1000 Faculty Rev-490. doi: 10.12688/f1000research.13674.1. Epub 2018 Apr 24
[PubMed PMID: 29770198]
Level 3 (low-level) evidence
[15]
Murrone D, De Paulis D, di Norcia V, Di Vitantonio H, Galzio RJ. Surgical management of falcine meningiomas: Experience of 95 patients. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2017 Mar:37():25-30. doi: 10.1016/j.jocn.2016.11.002. Epub 2016 Nov 22
[PubMed PMID: 27884604]