Introduction
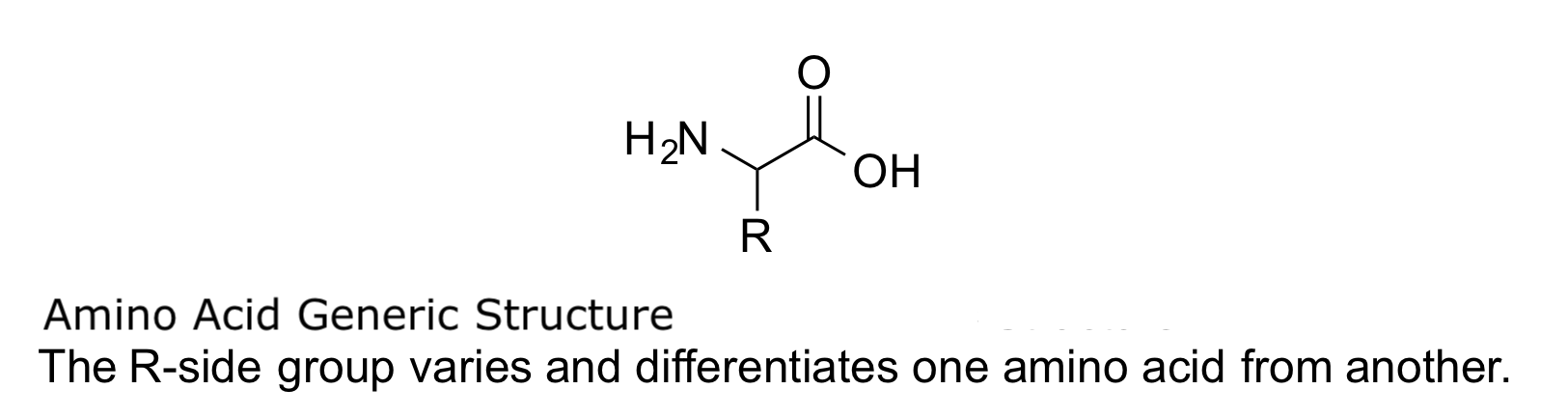
Proteins are made up of 20 amino acids. Each amino acid has an α-carboxyl group, a primary α-amino group, and a side chain called the R group (see Image. Amino Acid Generic Structure). Unlike other amino acids, proline has a secondary amino group. The side chain varies from one amino acid to the other. Nutritionally, amino acids are divided into 3 groups—essential, nonessential, and semi-essential. Semi-essential amino acids are synthesized by the body but are designated essential during periods of stress.
Nine amino acids, including histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine, are classified as essential amino acids because they cannot be synthesized by human or other mammalian cells. Therefore, these amino acids must be supplied from an exogenous diet. Non-essential amino acids not primarily derived from the diet are synthesized by the body. Semi-essential amino acids are growth-promoting amino acids. They are essential in growing children, pregnant women, and lactating women. Amino acids are further classified into 3 groups depending on the structure of the R-group—neutral, acidic, and basic. In addition to serving as the building block of proteins and peptides, amino acids play crucial roles in various important functions.
Some amino acids are converted to carbohydrates and are called glucogenic amino acids. Certain amino acids give rise to specialized products. For example, tyrosine can be converted to hormones, such as thyroid hormones, epinephrine, norepinephrine, and melanin. Methionine, in its active form known as S-adenosylmethionine, plays a critical role in cellular processes by transferring the methyl group to various substances through a process called transmethylation. Cystine and methionine are the primary sources of sulfur.
Besides 20 amino acids that participate in protein synthesis, recently, 2 more new amino acids have been described—selenocysteine and pyrrolysine. Selenocysteine occurs at the active site of several enzymes, including thioredoxin reductase and glutathione peroxidase. Pyrrolysine is not present in humans but is used in the biosynthesis of proteins in some methanogenic species, such as archaea and bacteria.[1][2][3]
Fundamentals
Amino acids are the fundamental building blocks of proteins and nitrogenous backbones for compounds such as neurotransmitters and hormones. In chemistry, an amino acid is an organic compound containing an amino functional group (-NH2) and a carboxylic acid functional group (-COOH). Proteins are long chains or polymers composed of a specific type of amino acid known as an α-amino acid. These α-amino acids are unique because their amino and carboxylic acid functional groups are separated by only 1 carbon atom, typically a chiral carbon. This activity focuses solely on the α-amino acids that constitute proteins.[4]
Proteins are chains of amino acids that assemble through amide bonds known as peptide linkages. The difference in the side-chain group or R-group determines the unique properties of each amino acid. The uniqueness of different proteins is determined by the amino acids they contain, the arrangement of these amino acids in a chain, and the complex interactions the chain makes with itself and the environment. These amino acid polymers give rise to the diversity observed in living organisms, collectively known as the primary, secondary, tertiary, and quaternary structures of the amino acids.
Approximately 20,000 unique protein-encoding genes are responsible for more than 100,000 unique proteins in the human body. Although hundreds of amino acids are found in nature, only about 20 are necessary to synthesize all the proteins in the human body and most other forms of life. These 20 amino acids are all L-isomer, α-amino acids. All amino acids, except for glycine, contain a chiral αcarbon. All these amino acids are L-isomers with an R-absolute configuration except for glycine and cysteine. Glycine lacks a chiral center, whereas cysteine has an S-absolute configuration due to its sulfur-containing R-group. Selenocysteine and pyrrolysine are considered the 21st and 22nd amino acids, respectively. More recently discovered amino acids may be incorporated into protein chains during ribosomal protein synthesis. Although pyrrolysine has biological functionality, humans do not incorporate it into protein synthesis. When translated, these 22 amino acids may also be modified through a post-translational modification to add further diversity in generating proteins.[5]
The 20 amino acids that comprise proteins include alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine.
Among these 20 amino acids, 9 are essential—phenylalanine, valine, tryptophan, threonine, isoleucine, methionine, histidine, leucine, and lysine.
The human body can synthesize dispensable amino acids, making them non-essential and unnecessary to include in a diet. For most physiological states in a healthy adult, the above 9 amino acids are the only essential amino acids. However, amino acids such as arginine and histidine may be considered conditionally essential because the body cannot synthesize them in sufficient quantities during specific physiological periods of growth, including pregnancy, adolescent growth, or recovery from trauma.[6]
Newly discovered amino acids include selenocysteine and pyrrolysine.
Molecular Level
Peptide bonds join together the 20 amino acids found in proteins. The linear sequence of the linked amino acids contains the information necessary to generate a protein molecule with a specific 3-dimensional shape. The sequence of amino acids in a protein is referred to as the primary structure of the protein. The sequence of nucleotides in a protein-coding region of the DNA specifies the amino acid sequence of a polypeptide.
The polypeptide backbone of proteins does not form a random 3-dimensional structure; instead, the regular arrangements of amino acids are located near each other in the linear sequence. These arrangements are commonly termed the secondary structure of the polypeptide or protein. The α-helix and β-sheet are examples of secondary structures normally found in proteins. The primary structure of a polypeptide chain determines the final tertiary structure, which refers to the folding of domains and the final arrangement of domains in the protein.
The amino acid sequence determines the unique 3-dimensional structure of each protein. Interactions between the amino acid side chains determine the folding of the polypeptide to form a particular structure. Various interactions stabilize the 3-dimensional structure of proteins, including disulfide bonds, hydrophobic interactions, hydrogen bonds, and ionic interactions. Many proteins consist of a single polypeptide chain and are described as monomeric proteins. However, others may consist of 2 or more polypeptide chains that may be structurally identical or unrelated. The arrangement of these subunits is termed the protein's quaternary structure. Subunits are held together by noncovalent interactions such as hydrogen bonds, ionic bonds, and hydrophobic interactions.
Protein denaturation can result in the unfolding and alteration of the protein's structure, a process that may be reversible but is more often irreversible. Diseases can arise when a normal protein adopts a cytotoxic conformation, as observed in Alzheimer's disease, transmissible spongiform encephalopathies, and Creutzfeldt-Jakob disease.[7][8][9] In Alzheimer's disease, the normal amyloid protein undergoes a unique conformational state that leads to the formation of neurotoxic amyloid protein assemblies consisting of β-pleated sheets. In transmissible spongiform encephalopathies, the infective agent converts the normal prion protein into the pathogenic conformation.[10][11]
Mechanism
Although human protein synthesis requires 20 amino acids, only about half of these required building blocks can be synthesized by humans. Humans and other mammals only have the genetic material required to synthesize the enzymes found in the biosynthesis pathways for non-essential amino acids. There is likely an evolutionary advantage behind removing the long pathways required to synthesize essential amino acids from scratch. By losing the genetic material required to synthesize these amino acids and relying on the environment to provide these building blocks, organisms can reduce energy expenditure, especially when replicating their genetic material. As a result, survival advantage is achieved, but dependency on other organisms for the essential materials is needed for protein synthesis.[12][13][14]
Clinical Significance
The classification of essential and nonessential amino acids was first reported in nutritional studies in the early 1900s. A study by Rose in 1957 found that the human body could stay in nitrogen balance with a diet of only 8 amino acids.[15] These 8 amino acids were the initial group classified as essential or indispensable. Subsequent feeding studies with purified amino acids allowed scientists to pinpoint essential amino acids accurately. These studies revealed that removing individual essential amino acids from the diet led to impaired growth or disrupted nitrogen balance in subjects. Further research found that certain amino acids are conditionally essential, depending on the subject's metabolic state. For example, although a healthy adult may synthesize tyrosine from phenylalanine, a young child may not have developed the required enzyme (phenylalanine hydroxylase) to perform this synthesis. Therefore, they would be unable to synthesize tyrosine from phenylalanine, making tyrosine an essential amino acid under those circumstances. This concept also appears in different disease states.
Deviations from the standard metabolic state of a healthy adult may place the body in a metabolic state that requires more than the standard essential amino acids to maintain nitrogen balance. Typically, the optimal ratio of essential and nonessential amino acids requires a balance dependent on physiological requirements that differ between individuals. Finding the optimal ratio of amino acids in total parenteral nutrition for liver or kidney disease is an excellent example of different physiological states requiring different nutrient intakes. Therefore, the terms essential and nonessential amino acids may be misleading as all amino acids may be necessary to ensure optimal health.
Clinical symptoms may appear during states of inadequate intake of essential amino acids, such as vomiting or low appetite. These symptoms may include depression, anxiety, insomnia, fatigue, weakness, and growth stunting in the young. These symptoms are mostly caused by a lack of protein synthesis in the body because of the lack of essential amino acids. Adequate amounts of amino acids are necessary to produce neurotransmitters and hormones, muscle growth, and other cellular processes. These deficiencies are typically present in poorer parts of the world or older adults with inadequate care. Alternatively, impaired amino acid metabolism is associated with various human diseases, including phenylketonuria, tyrosinemia, homocystinuria, non-ketotic hyperglycinemia, and maple syrup urine disease.[16][17][18][19][20][21]
Kwashiorkor and marasmus are examples of more severe clinical disorders caused by malnutrition and inadequate intake of essential amino acids as a macronutrient. Kwashiorkor is a form of malnutrition characterized by peripheral edema, dry peeling skin with hyperkeratosis and hyperpigmentation, ascites, liver malfunction, immune deficits, anemia, and relatively unchanged muscle protein composition. The diseases result from a diet with insufficient protein but adequate carbohydrates. Marasmus is a form of malnutrition characterized by wasting caused by inadequate protein and caloric intake.[22]