Introduction
The mammary gland is a highly evolved and specialized organ developing on each side of the anterior chest wall. This organ's primary function is to secrete milk. Though the gland is present in both sexes, it is well-developed in females but rudimentary in males. The mammary gland is a vital accessory organ in the female reproductive system.
The mammary gland is classified as apocrine. Thus, the secretory cells' apical segment and a portion of their cytoplasm become part of the secretion. The mammary gland usually weighs between 500 and 1000 grams each. The organ is hemispherical in young adult females but becomes pendulous later in life. This article discusses the anatomy, function, and clinical importance of the mammary gland.
Structure and Function
Structure
The mammary gland is situated in the pectoral region in the superficial fascia. However, a segment called the "axillary tail of Spence" pierces the deep fascia and lies in the axilla up to the 3rd rib level. The mammary gland extends vertically from the 2nd to the 6th rib. Horizontally, it spreads from the lateral sternal border to the mid-axillary line.
Deep to the mammary gland tissue is the retromammary space, a loose connective-tissue plane that gives free mobility to the gland. Below the retromammary space is the pectoral fascia, which covers the pectoralis muscle. The serratus anterior and external oblique are other muscles that lie deep in the mammary gland.
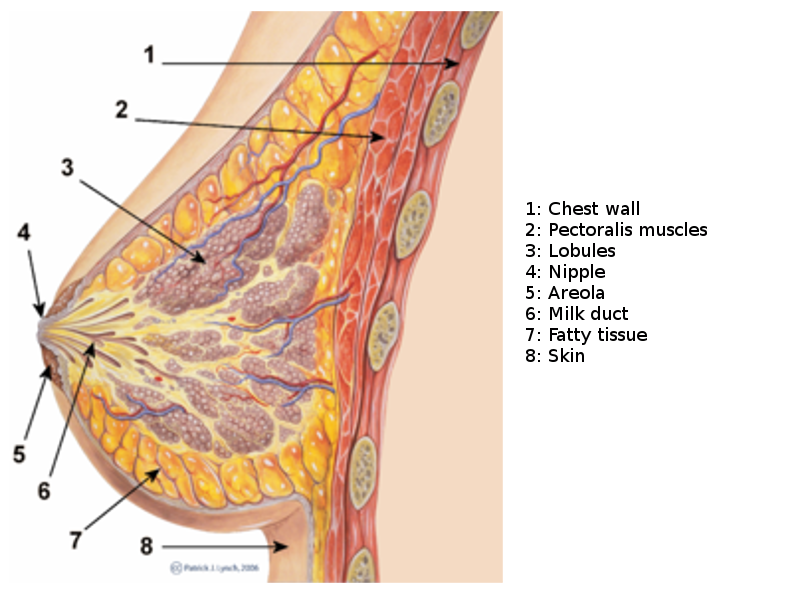
The mammary gland is divided into 3 parts: skin, parenchyma, and stroma (see Image. Breast Sagittal View).
Skin
The skin consists of the nipple and areola. The nipple is a conical eminence situated in the 4th intercostal space (ICS). Piercing the nipple are 15 to 20 lactiferous ducts. The nipple contains richly innervated circular and longitudinal smooth muscle fibers, which make it erect upon stimulation. The nipple usually has no sweat glands, fat, or hairs.
The areola is the dark pinkish-brown area around the nipple. This area is rich in modified sebaceous glands (tubercles of Montgomery) during pregnancy and lactation. These modified glands produce oily secretions that prevent nipple and areolar cracking. Notably, the areola is devoid of fat and hair.
Parenchyma
Glandular tissue is comprised of branching ducts and terminal secretory lobules. One lactiferous duct drains 15 to 20 lobes. These ducts enlarge to form the lactiferous sinus before they open separately into the nipple. Milk collects in the lactiferous sinuses and is released in response to the baby's suckling. The lactiferous ducts are arranged radially in the nipple. Hence, incisions in this area must be oriented radially to avoid cutting through multiple lactiferous ducts.
Stroma
The stroma is the supporting framework of the breast parenchyma. The stroma has fibrous and fatty areas.
Fibrous stroma gives rise to septa called "suspensory ligaments of Cooper," which separate the lobes and suspend the mammary gland from the pectoral fascia. In patients with breast cancer, contraction of these ligaments causes breast rigidity and puckering of the overlying skin. In breast cancer, the Peau d'orange sign arises from lymphatic obstruction and subsequent cutaneous edema and fibrosis.
The bulk of the mammary gland is filled with variable amounts of stromal fat.
Mammary Gland Quadrants
The mammary gland is best examined by dividing it into 4 quadrants, with vertical and horizontal imaginary lines passing through the nipple (see Image. Mammary Gland Quadrants). The regions are designated as the upper outer (UOQ), upper inner (UIQ), lower inner (LIQ), and lower outer (LOQ) quadrants.[1][2]
Function
The primary purpose of the mammary gland is to secrete milk for infant breastfeeding. This organ also plays an essential role in female sexuality.
Breast development is minimal and comparable in both sexes until females reach puberty. Estrogen, progesterone, and growth hormone spurt during the pubertal phase and induce greater development in females than males. The smooth contour of female breasts is due to increased adipose tissue.
The female breasts further increase in size in early pregnancy, with estrogen and progesterone inducing parenchymal growth and ductal branching. Secretory alveoli and the surrounding connective tissue start developing at duct terminals. In the later stages of pregnancy, these alveoli are filled with milk under prolactin's influence. When lactation stops, the secretory alveoli shrink, decrease in number, and disappear. However, the mammary gland never returns to prepubertal form. After menopause, the breasts regress in size due to diminishing circulating estrogen levels.
Embryology
Genetic and hormonal factors influence ectodermal cells to form the human breasts starting in the 4th week of embryonic life. Ectodermal thickenings, known as mammary ridges (milk ridge or milk line), emerge on the chest around the 4th intercostal space, giving rise to rudimentary mammary buds by the 5th week of gestation. These primary mammary buds extend downward, developing into secondary buds and intricate mammary lobules over the next 7 weeks. The breast stroma, fat, ligaments, nerves, arteries, veins, and lymphatics undergo development throughout the gestational period.
Beyond the 12th week, the secondary buds continue their growth, elongating and branching into 15 to 20 solid cords that differentiate into the lactiferous breast ducts and their branches. The ducts later canalize under the influence of maternal sex hormones, connecting the developing nipple to the expanding mammary lobules.
The fetal nipple is inverted but everts at birth due to the proliferation of its modified sebaceous glands (future Montgomery glands) and muscle tissue. Areolar pigmentation also increases at birth.[2]
Blood Supply and Lymphatics
Arterial Supply
The following arteries supply the mammary glands:
- The internal thoracic artery's perforating branches from the 2nd to the 6th ICS provide circulation to the medial gland regions.
- The lateral thoracic artery supplies the superolateral breast parenchyma.
- The axillary artery's superior thoracic, thoracoacromial, subscapular, and thoracodorsal branches supply a portion of the superior breast parenchyma.
- The musculophrenic artery originates from the internal thoracic artery and supplies inferior breast segments.
- The branches of the anterior and posterior intercostal arteries penetrate the chest wall muscles and supply the deep central breast parenchymal tissues.
- Lateral intercostal arteries travel alongside the pectoralis and serratus anterior muscles and send perforating branches into the deep breast parenchymal tissues.
Venous Drainage
Breast veins are divided into superficial and deep veins. Superficial veins commonly drain the central and peripheral breast areas. The central veins form a venous plexus known as the "circulus venosus of Haller." Blood flows from this venous network into the internal thoracic vein medially, lateral thoracic veins laterally, and the superficial neck veins superiorly. The deep breast veins drain into the internal thoracic, axillary, and posterior intercostal veins. Valves are typically absent in breast veins, and intramammary venous anastomoses are frequently observed.
Lymphatic Drainage
Principal Lymph Nodes Draining the Breasts
Lymph from the breasts drains primarily toward the axillary and internal mammary groups, though a small amount also empties to nearby lymph nodes. These lymph nodes are clinically important, as they are common sites for breast cancer metastasis.[3][4]
- Axillary nodes: The axillary lymph nodes are located in 5 regions in the axillary fat pad. The axillary nodes include the following:
- Pectoral (Anterior or External): This lymph node group lies on the inferior pectoralis minor border along the lateral thoracic vessels and drains the outer quadrants of the breast.
- Subscapular (Posterior or Scapular): This group lies on the axilla's posterior wall along the lower subscapularis margin. The subscapular nodes drain the LOQ.
- Humeral (Lateral): This lymph node group lies over the axilla's lateral wall beside the humerus and drains minimal lymph from the breast.
- Central: This group lies in the axillary base and receives lymph from the anterior, posterior, and lateral lymph node groups.
- Apical (Subclavicular): This lymph node group lies deep in the axilla's apex and receives lymph from all the above lymph nodes. The apical lymph nodes also directly drain the UIQ.
- Interpectoral (Rotter nodes): This group lies between the pectoralis major and minor—the muscles posterior to the breast. These nodes drain the breasts directly. Tumor spread to the Rotter nodes may cause breast cancer recurrence, as these nodes may be missed during surgery.[44]
- Internal mammary (parasternal) nodes: These lymph nodes are situated lateral to the sternum's lateral border, surrounding the internal mammary artery. Some cross to the contralateral side and drain to the contralateral group of parasternal nodes. These nodes drain the inner breast quadrants. This route is often where cancer from one breast metastasizes to the other and becomes bilateral.
The axillary lymph node group is the main breast drainage site, collecting 75% to 80% of lymph from the breasts. The pectoral group drains most of the lymph going to the axillary nodes. The remaining 20% to 25% of breast lymph drains to the internal mammary nodes.
Other Lymph Nodes Draining the Breasts
A small amount of breast lymph drains into the following nodes:
- Supraclavicular nodes
- Cephalic (deltopectoral) nodes
- Posterior intercostal nodes
- Subdiaphragmatic and subperitoneal lymph plexuses
The subdiaphragmatic and subperitoneal lymph plexuses are the routes where breast cancer metastasizes toward the abdomen.
Nerves
The literature has reported vast differences in breast tissue innervation, especially in the areola and nipple. However, most agree that the anterior and lateral cutaneous branches innervate the mammary gland from the 2nd to 6th intercostal and supraclavicular nerves. These nerves form a plexus deep into the areola in the subdermal tissue and supply the nipple and areola.[5][6] The nipple and areola are highly sensitive to touch, and these sensations are carried mainly by the T4 spinal nerve dermatome.
The breast's nerve supply, particularly in the nipple and areolar areas, is the subject of recent studies. Innervation in these regions must be preserved during breast surgical reconstruction.[7][8][9]
Muscles
The mammary gland is a modified sweat gland comprised of parenchymal and stromal tissues covered by skin. As previously mentioned, the nipple area becomes erect when stimulated due to its smooth muscle fibers. Besides these muscles, chest wall skeletal muscles are situated deep to the breasts.
The pectoralis muscle is the predominant skeletal muscle posterior to the mammary gland. The pectoral fascia covers this muscle. The pectoralis minor lies posterolateral to the pectoralis major. Other skeletal muscles deep into the breasts include the serratus anterior and external oblique.
The skeletal muscles in the mammary region are surgically important. Successful breast reconstruction and implantation require an intact pectoralis muscle.[10][11] Meanwhile, the muscle flap most frequently used for breast reconstruction is the latissimus dorsi.[12][13]
Physiologic Variants
The mammary gland shows physiological variations in size, shape, contour, density, spacing, and volume. These variations depend on factors like age, height, weight, genetic composition, race, nourishment, and environment. Breast asymmetry is observed in 25% of females, with the size and shape differing in the same person.
Nipple inversion is also quite frequently seen in females. This condition can be physiological and correct spontaneously over time or during pregnancy. However, nipple inversion may also be an early indicator of underlying pathology.[14]
Surgical Considerations
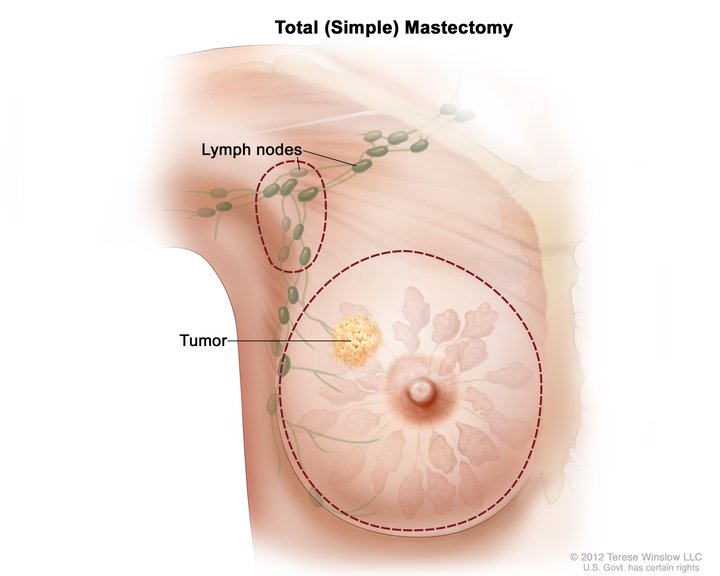
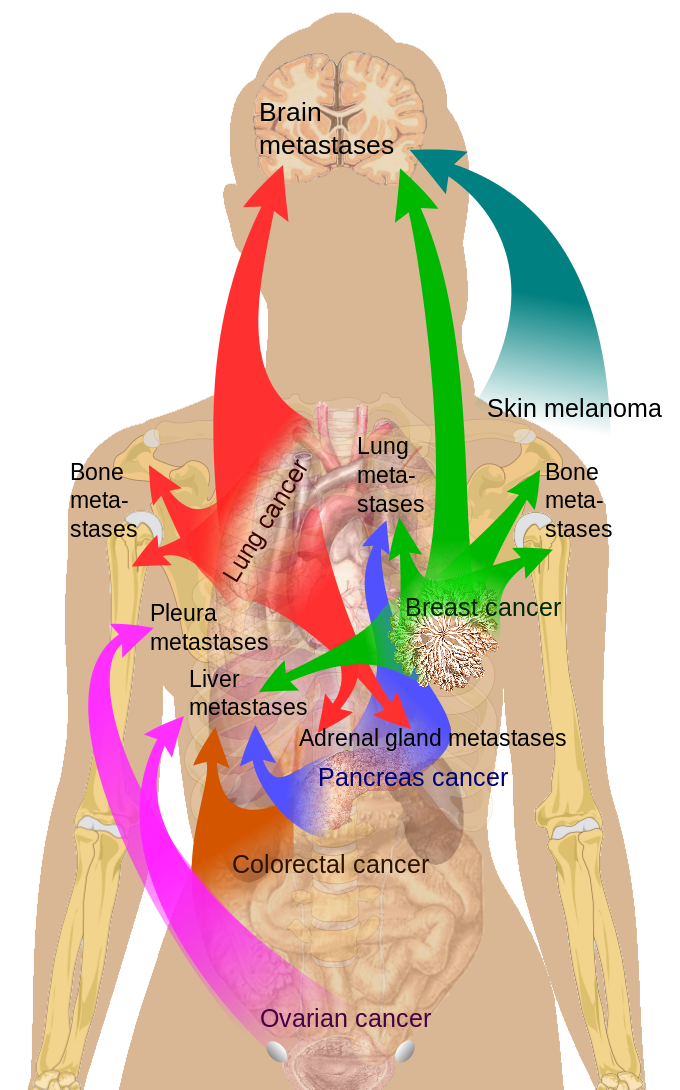
Surgery, ranging from lumpectomy to radical mastectomy, is the treatment of choice for breast lesions (See Image. Total Mastectomy). As mentioned earlier, breast malignancies can metastasize through the lymphatics (see Image. Cancer Metastasis Sites). Knowledge of the mammary glands' lymphatic drainage helps surgeons identify the affected lymph nodes before dissection.[15]
Recurrence is common, making adjuvant radiotherapy necessary after breast cancer surgery. Patients with a higher recurrence risk may also receive adjuvant chemotherapy, which may prolong their disease-free overall survival period. Hormonal therapy is helpful when breast tumors are estrogen- and progesterone-receptor-positive. Tamoxifen has been proven effective in estrogen-positive breast cancer patients.
The global rise in breast carcinoma cases has resulted in an increase in surgical mastectomy and demand for surgical breast reconstruction, breast implantation, and mammoplasty.[16][17] This trend has resulted in increased interest in understanding breast anatomy and developing new surgical breast treatments.[18][19][20]
Clinical Significance
Breast lesions may be benign or malignant. Most breast lesions are benign, but they have gained little attention because they are usually non-fatal.[21] Fibrocystic changes are the most common benign lesions, constituting almost 40% of all cases, even during the premenopausal years.
Fibrocystic breast changes occur in the duct and lobular epithelia and are classified into proliferative and nonproliferative. Examples of nonproliferative lesions are cystic and fibrotic changes. Epithelial hyperplasia and sclerosing adenosis are examples of proliferative breast changes.
Fibroadenomas are benign stromal tumors constituting 7% of all breast lesions. These outgrowths are more common in young adult women than menopausal women. Fibroadenomas are hormonally responsive, developing during pregnancy and late menstrual cycle stages and regressing after menopause. The phyllodes tumor and intraductal papilloma are other common benign neoplasms.
Breast cancer has generated significant concern worldwide, as it is the most common malignancy after lung cancer and a leading cause of cancer-related deaths among women.[22][23] Breast carcinoma may also affect males, though the risk is lower.[24] The UOQ is the site most commonly affected by breast carcinoma, seen in almost 60% of the cases.[25]
Breast cancer risk factors include age older than 40 years, nulliparity, late first pregnancy, oral contraceptive use, obesity, high-fat diet, positive family history, genetic factors, alcohol consumption, cigarette smoking, and ionizing radiation exposure. Breast cancer is more common in Western countries. Afro-American females have a higher breast cancer risk than Asians and Africans.[26][27]
Malignant lesions are classified into invasive and noninvasive types. Noninvasive types include ductal carcinoma in situ (DCIS), also known as Paget disease of the nipple, and lobular carcinoma in situ (LCIS). Invasive or infiltrative carcinomas have many types, including invasive ductal carcinoma, invasive lobular carcinoma, medullary carcinoma, colloid carcinoma, and tubular carcinoma. Invasive ductal carcinoma accounts for 70% of breast malignancies. A rare mammary gland neoplasm is myoepithelioma, which is diagnosed mainly by immunohistochemical and histological features.[28]
Cancer cells may spread to the spinal cord via the communication between the valveless deep breast veins and the Batson plexus in the vertebral area. Vertebral metastasis can cause spinal fractures that can injure the cord[29]
The prognosis of breast carcinoma in females depends on tumor size, lymph node involvement, tumor grading, the presence of metastasis, and structural features (see Image. Metastasis Sites).[23] Screening helps improve breast cancer prognosis as it increases the likelihood of early detection.
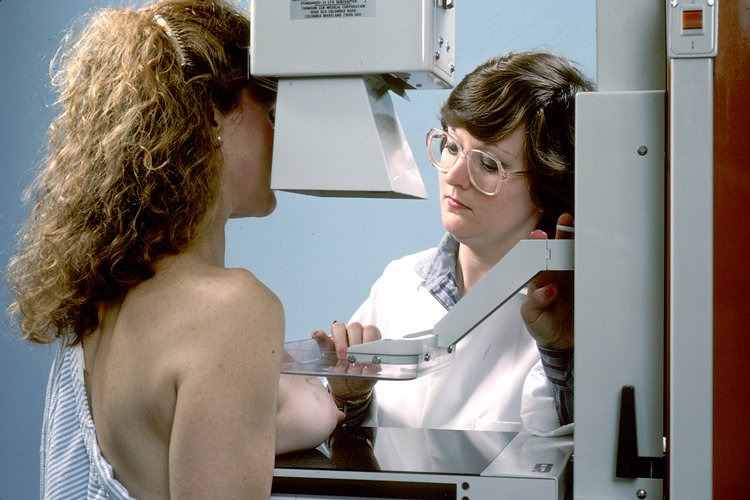
Breast self-examinations increase the likelihood of detecting breast cancer early.[45] Imaging studies used for breast cancer screening include mammography and infrared breast thermography (see Image. Mammogram).[30] In patients younger than 40 years, mammography's sensitivity in detecting breast tumors is low due to high breast tissue density. Ultrasound is the recommended modality in these patients. In individuals who previously had breast implants, magnetic resonance imaging (MRI) is the recommended screening method for breast cancer.[46] A biopsy confirms the diagnosis.[31][32]
Other Issues
Common Congenital Anomalies
Common congenital anomalies include the following:[33][34][33]
- Ectopic breast: The mammary gland develops away from its usual site. Ectopic breasts may appear on another part of the chest or outside the thorax.
- Polymastia: The growth of more than one breast on each side
- Polythelia: Multiple nipples, usually 2 or 3, grow on a single breast. Often, one of them is functional, while the others are rudimentary. Polymastia and polythelia combined occur in 0.2% to 5.6% of women.
- Micromastia: The mammary gland tissue fails to develop fully and remains small.
- Macromastia: The mammary gland grows much more than the usual size and hypertrophies.
- Gynecomastia: Breast enlargement in males, though the glands remain non-functional. About 80% of patients with Klinefelter syndrome have gynecomastia.
- Retracted or inverted nipple: Incomplete nipple development, typically linked to fibrous nipple tethering within a hypoplastic ductal system
Of these mammary gland conditions, the most common is the ectopic breast.
Other Breast Disorders
Other conditions involving the breasts include the following:
Congenital Hypoplastic Breast Anomalies
Three conditions fall under this category: athelia, amazia, and amastia. Athelia is the absence of the nipple-areolar complex. Amazia is the absence of breast glandular tissue. Amastia is the absence of both the nipple and glandular tissue. These conditions can manifest unilaterally or bilaterally. Congenital breast hypoplasia is typically closely linked to a congenital syndrome or deformity, most commonly ectodermal dysplasia.[35]
Acquired Hypoplastic Breast Anomalies
Acquired hypoplastic breast anomalies include hypomastia and hypoplasia. These conditions can stem from hormonal irregularities, leading to insufficient breast development during puberty. Secondary breast underdevelopment may also arise from iatrogenic causes. Surgical procedures, like chest tube thoracostomy during the early stages of breast development, can cause scarring that impedes normal postpubertal growth.[36]
Poland Syndrome
This congenital condition presents with the absence or underdevelopment of the pectoralis major and associated abnormalities of the pectoralis minor, sternum, ribs, and upper limbs. The breast on the ipsilateral side may also be underdeveloped, though this anomaly may not be clinically evident until after the onset of thelarche.[37][38]
Endocrine Abnormalities
Endocrine disorders affecting breast tissue development include Turner, Noonan, and Van Maldergem syndromes. These conditions are commonly associated with a lack of postpubertal breast development.[39][40][41]
Stenotic Breast (tuberous malformation)
A stenotic breast has an irregular shape and growth pattern, resembling a tuberous plant's root. This condition leads to areolar enlargement and intermammary cleft widening.[42]
Mammary Gland Tuberculosis
Tuberculous mammary gland infection is rare in developed countries, with an incidence of 0.1% of all breast lesions. This condition is more common in developing countries.[43]