Continuing Education Activity
Necrotizing periodontal diseases (NPDs) encompass necrotizing gingivitis (NG), necrotizing periodontitis (NP), necrotizing stomatitis (NS), and in extreme cases, NPDs can lead to noma (cancrum oris). These are all considered stages of the same condition due to their shared etiology and clinical presentation. The most typical features of NPDs are pain, gingival necrosis, interdental ulceration, and in more advanced stages, osteonecrosis. This activity outlines the etiology, evaluation, and treatment of necrotizing periodontal diseases, particularly necrotizing gingivitis and necrotizing periodontitis. It highlights the role of the healthcare team in identifying patients at risk for and managing patients currently with this condition.
Objectives:
- Review the etiology of necrotizing periodontal diseases.
- Describe the presentation of a patient with necrotizing periodontal diseases.
- Summarize the management of a patient with necrotizing periodontal diseases.
- Outline the importance of improving care coordination among the interprofessional team to enhance care delivery for patients with necrotizing periodontal diseases.
Introduction
Necrotizing periodontal diseases (NPDs) encompass necrotizing gingivitis (NG), necrotizing periodontitis (NP), necrotizing stomatitis (NS), and in extreme cases, NPDs can lead to noma (cancrum oris). These are all considered stages of the same condition due to their shared etiology and clinical presentation.[1] The most typical features of NPDs are pain, gingival necrosis, interdental ulceration, and in more advanced stages, osteonecrosis.[2]
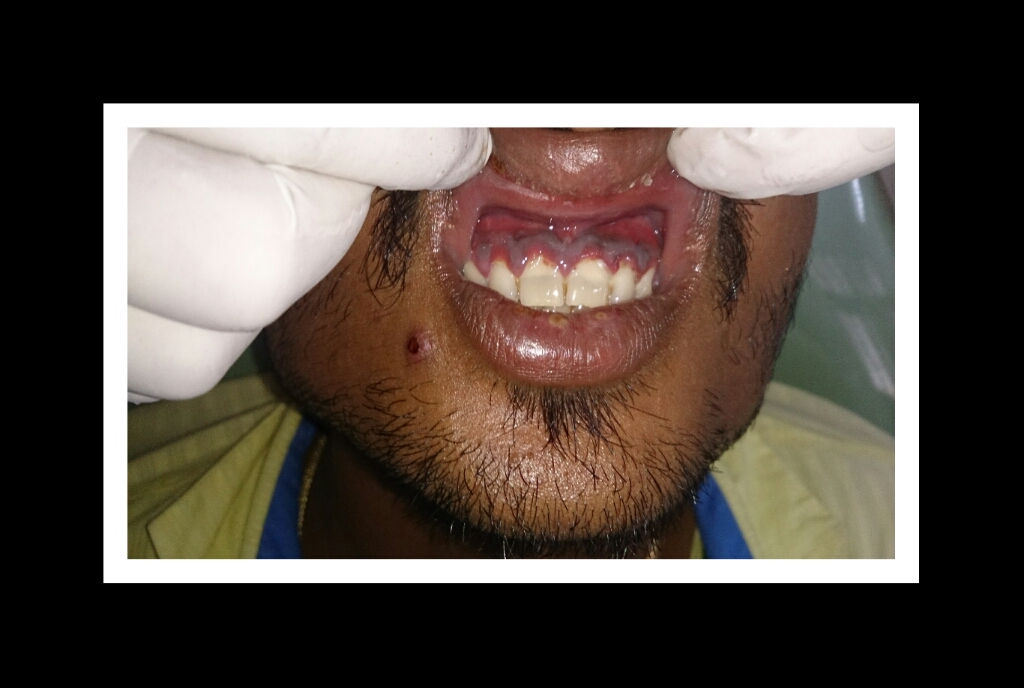
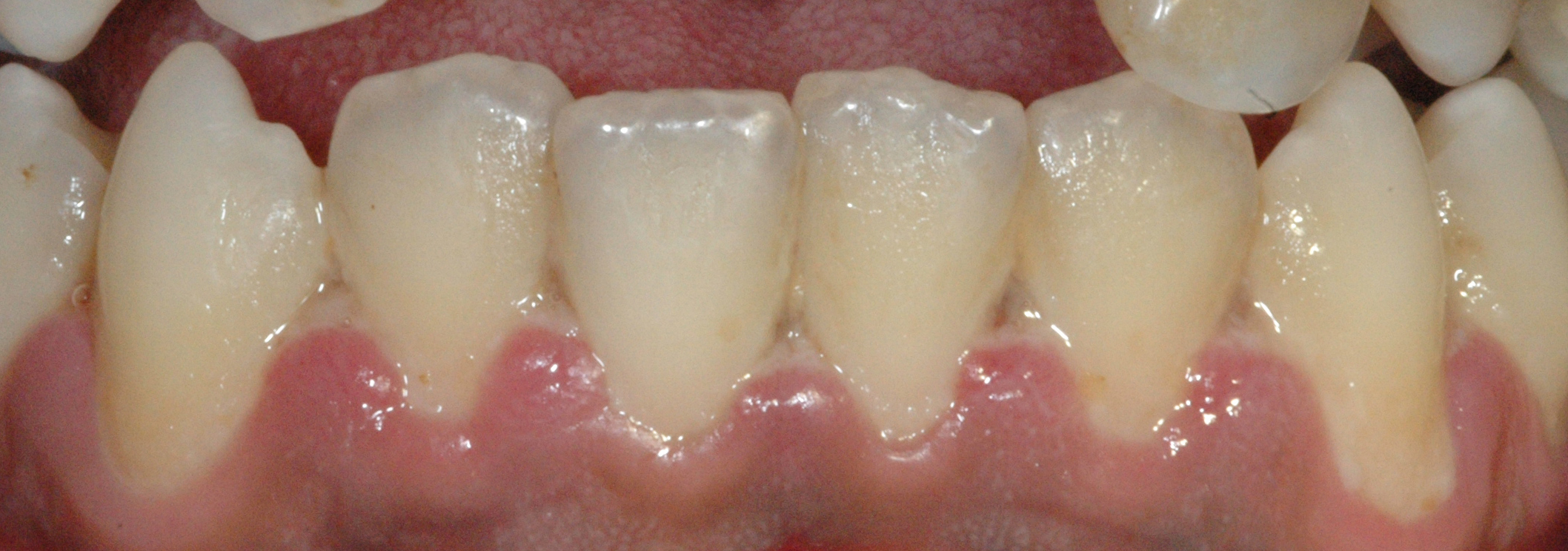
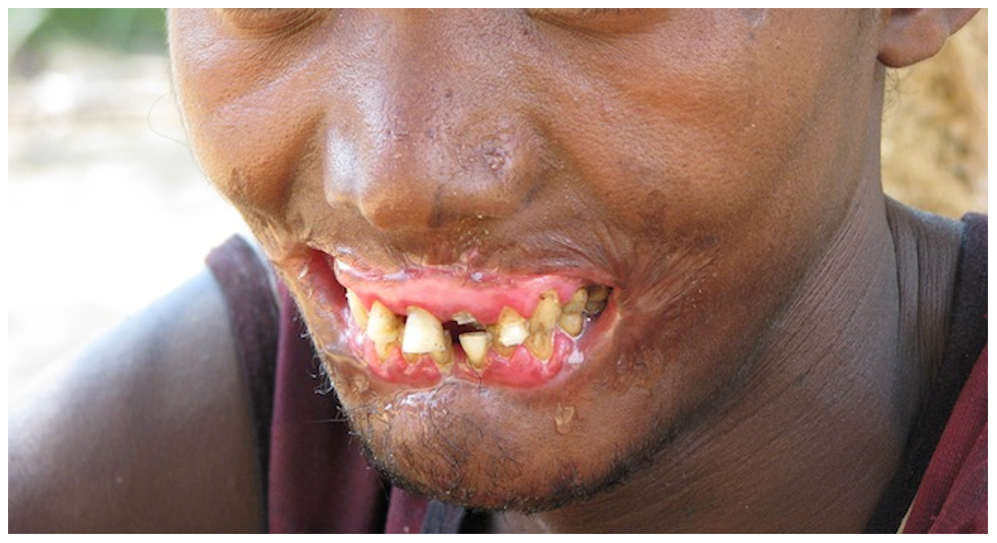
Necrotizing gingivitis is the most common form and progresses to necrotizing periodontitis in susceptible populations, such as in immunosuppressed patients. The necrotic destruction is limited to the interdental papillae that exhibit the typical punched-out appearance.[2] No loss of attachment has occurred, and at this point, the disease is reversible upon treatment. Necrotizing gingivitis may progress to rapid periodontal loss, pseudomembrane formation, and systemic symptoms like fever, pyrexia, and lymphadenopathy.[2] This stage is known as necrotizing periodontitis. Although uncommon, the disease can then spread to affect alveolar bone with osteonecrosis and sequestration, receiving the name necrotizing stomatitis.[2] The most severe form of NPDs is noma, a fast-spreading noncontagious orofacial gangrene affecting malnourished children from undeveloped countries, mainly in tropical regions like sub-Saharan Africa.[3] Noma rapidly compromises the soft and hard tissues of the face and can be fatal.[3]
Etiology
Although necrotizing periodontal diseases are infectious conditions, immunosuppression is the most critical predisposing factor. Cases of necrotizing gingivitis and necrotizing periodontitis are almost always found in patients with an immunosuppressed condition, most notably HIV. A CD4 count of less than 200 in an HIV-positive patient is more strongly linked to necrotizing periodontitis than any other risk factor.[4][5][4] But, other diseases such as leukemia, neutropenia, diabetes mellitus, and long-term immunosuppressant therapy have also been noted.[6][7][8]
Other contributing factors include tobacco smoking, unusual psychological stress, severe malnutrition, poor sleeping habits, and poor oral hygiene. Most cases of NPDs are seen in patients that are tobacco smokers. Malnutrition has been shown to be an important risk factor. In developed countries, NPDs are associated with populations who display poor eating habits, such as college students, whereas, in developing countries, they are associated with young children with deficient protein intake.[9] Also, patients with a history of necrotizing gingivitis are considered at increased risk of developing necrotizing periodontitis.[4]
Virulent bacteria, specifically Fusobacteria, Prevotella intermedia, Porphyromonas gingivalis, and Treponema sp, have been identified in the necrotic lesions associated with NPDs.[4] Additionally, antibiotics have been shown to alleviate symptoms in the acute phase, indicating a bacterial involvement in the disease process. However, it is unclear whether these organisms are the causative agent in the disease process or if they represent a secondary overgrowth due to the immunosuppressed condition. Furthermore, the disease is not known to be transmissible, indicating that the etiology is not due to exogenous factors but rather due to preexisting host factors.[8]
A limited understanding exists surrounding the actual cause of necrotizing periodontal diseases; however, evidence points to an existing periodontal condition that is given the opportunity to proliferate into a more rapid and destructive state.[10]
Epidemiology
The worldwide prevalence of necrotizing periodontal diseases is less than 1%.[2] The most affected age group is young adults aged 18 to 30, malnourished children, or immunocompromised patients.[2] NPDs are more commonly found in countries with lower socioeconomic status and are rarely seen in developed countries.[2]
A major epidemiologic risk factor is malnutrition. NPDs are most commonly associated with very young children in developing countries due to severe malnourishment, specifically a low protein intake.[11] Additional risk factors most widely associated with emerging countries include tobacco smoking and poor oral hygiene.[12]
Pathophysiology
The exact pathogenesis of necrotizing periodontal diseases is not fully understood. However, the initial stage of NG is believed to be caused by commensal oral organisms that become pathogenic in response to reduced host response.[13] Fusobacteria and spirochete species have commonly been implicated in NPDs; however, it is unclear whether these are causative organisms or represent a secondary or opportunistic infection due to the patient's immunosuppressed condition.[14] These bacteroids, in addition to other primarily gram-negative species, produce a vast array of metabolites (collagenases, endotoxins, hydrogen sulfide, and fibrinolysin) responsible for the destruction seen in NPDs: rapid destruction of the periodontium, including the gingiva, periodontal ligament, and alveolar bone.[8][15]
Histopathology
In 1965, Listgarten provided electron microscopy (EM) data of the invasion of spirochetes in what was previously referred to as necrotizing ulcerative gingivitis (NUG) and differentiated between various zones within NUG. The zones are listed in order of increasing depth.
- Bacterial zone
- Neutrophil-rich zone
- Necrotic zone
- Zone of spirochete infiltration
The most superficial bacterial zone contains a variety of bacterial species. The neutrophil-rich zone contains various leukocytes, with the most prominent cell type being the neutrophil. In the next deeper zone, the necrotic zone, an abundance of dead cells and fragmented connective tissue can be found. Up until this final zone, a variety of bacteria species could be found, including spirochetes. However, in the zone of spirochete infiltration, spirochetes are the sole organism present and can be found in the deep, preserved tissue.[16]
History and Physical
As previously mentioned, necrotizing periodontal diseases are almost always found in patients with immunosuppression or psychological stress.[4] A history of tobacco smoking is a significant risk factor for developing an NPD.
Patients will usually report excruciating pain, fetid oral odor, and rapid onset of the condition, characteristic of necrotizing periodontal diseases - necrotizing periodontitis can lead to loss of periodontal attachment in as short as a few days.[4][17] In some NPDs, systemic involvement can be seen via swelling of regional lymph nodes. Fever and malaise are associated with more advanced stages of NPDs, e.g., necrotizing periodontitis.[17] NPD lesions are extremely painful, which provides a good differentiating feature from regular periodontal diseases that do not elicit pain. As a result of the pain, patients tend to stop their oral hygiene practices, contributing to halitosis. Additionally, patients may decrease their drink and food intake, potentially becoming malnourished, exacerbating symptoms of fever or malaise.[18]
The most typical clinical sign of NPDs is tissue necrosis, seen as ulcerated and necrotic gingiva, with a distinctive “punched-out” or “cratered” appearance.[15][18] These necrotic gingival tissues are covered by a pseudomembrane containing plasma proteins and white blood cells. When NG progresses to NP, loss of periodontal ligament and alveolar bone occurs. A linear erythematous area demarcates the ulcerated zone from the attached gingiva, alveolar mucosa, and free gingiva.[4] Provoked or spontaneous bleeding may be evident. Loss of attachment is the key feature of necrotizing periodontitis; however, deep periodontal pockets are uncommon (junctional epithelium is necrotized).[4] The disease progresses so rapidly that there is little time for pocket formation.[10][15] In necrotizing stomatitis, there is a destruction of the oral mucosa beyond the mucogingival junction and bone exposure, with alveolar bone necrosis and possible sequestration.[3][4]
Evaluation
NPDs are a clinical diagnosis, and intraoral examination should be accompanied by extraoral examination searching for lymphadenopathy or facial asymmetry [4]. A biopsy is unhelpful, as it will present as non-specific inflammation. Radiographs can be used to demonstrate the extent of alveolar bone loss.
On radiographic examination, the bone may show extensive changes or very little. Osteonecrosis of the bone may be seen as “moth-eaten” patchy radiolucency, resembling other conditions such as medication-related osteonecrosis of the jaw, osteoradionecrosis, and osteomyelitis.[2] Radiopaque areas corresponding to necrotic bone sequestra may be visualized. CT scans may be more helpful in differentiating between different conditions of the bone. But ultimately, elements of the history and clinical examination will determine a definitive diagnosis.[2]
Blood tests are recommended to investigate predisposing illnesses such as leukemia, neutropenia, and agranulocytosis.[18][9]
Treatment / Management
Necrotizing gingivitis and necrotizing periodontitis are mainly managed using local measures. The treatment of NPDs depends on the extent and severity of the disease, but in general, initial management includes:
- Gentle mechanical debridement under local anesthesia,
- Removing pseudomembrane with the help of a cotton pellet embedded in 0.12% chlorhexidine,
- Providing oral hygiene instructions
- Indicating either a 0.12% chlorhexidine mouthwash or hydrogen peroxide mouth rinse[4]
The use of nonsteroidal anti-inflammatory drugs (NSAIDs) as needed should be advised to the patient to help alleviate the pain.[4] Antibiotics are recommended as an adjuvant to local measures when there is evidence of systemic involvement, e.g., pyrexia, malaise, and lymphadenopathy.[19][4] Metronidazole is the antibiotic of choice due to the anaerobic bacteria involved in the condition. Amoxicillin is another option when metronidazole is contraindicated.
Modifying risk factors and treating underlying systemic conditions are key in managing NPDs. Tobacco smoking, psychological stress, and malnutrition should be managed to reduce the risk of future disease.[9][14] Patients with NP may require a medical consultation as the patient may have an underlying immunocompromising condition like HIV. These underlying or concurrent conditions need to be treated simultaneously with the required dental therapy.
Further advice may include taking vitamin supplements, avoiding spicy food, observing appropriate fluid intake, and improving sleep.[4] Finally, reviewing for further treatment and maintenance is vital in improving the disease prognosis.
Necrotizing periodontitis is a disease of tissue destruction of both soft tissue (gums) and hard tissue (alveolar bone). As a result of the disease, both soft and hard tissue defects can make oral hygiene difficult. After completing the acute treatment phase, patients may undergo treatments to surgically correct any defects, such as a gingivoplasty for smaller soft tissue defects or osteoplasty to repair larger, bony defects.[4]
Differential Diagnosis
Necrotizing periodontal diseases should be differentiated from the following:
- Oral mucositis
- HIV-associate periodontitis
- Herpes simplex virus (HSV)
- Scurvy
- Gingivostomatitis
- Desquamative gingivitis
- Invasive fungal disease
- Illicit-drug related gingival disease
- Agranulocytosis
- Leukemia
- Chronic periodontitis
Appropriate testing and history taking must be performed for an accurate diagnosis.[20][17]
Staging
According to the 2017 classification system put forth by the American Academy of Periodontology, necrotizing periodontal disease is a category of diseases that affects the periodontium through the extent of ulceration and necrosis. Within this group, there are three major subsets:
- Necrotizing gingivitis
- Necrotizing periodontitis
- Necrotizing stomatitis
The differences between these subsets are based on the extent and site of damage. Necrotizing gingivitis is used to describe necrosis and ulceration limited to the gingiva (gums). A progression of that condition is necrotizing periodontitis, where there is damage to and loss of the periodontium surrounding each tooth, including the gingival tissues, alveolar bone, and periodontal ligament. Lastly, necrotizing stomatitis is a term used to describe the destruction of the mucous membranes in the mouth and the areas beyond the mucogingival junction, such as the cheek, tongue, and palate.[21]
Prognosis
Recurrence of necrotizing periodontal diseases is not uncommon, and therefore regular recalls are recommended to monitor for signs of future illness. Additionally, a critical factor in the prognosis of NPDs is the management of risk factors. The prognosis is more favorable if patients improve their risk factors such as oral hygiene, nutrition, psychological stress, or smoking cessation. Additionally, treatment of underlying conditions, such as therapy for HIV, may reduce the patient's immunosuppressed state, thus improving their prognosis.[7][17]
Complications
There are several noted complications of necrotizing periodontal diseases, most of which are related to the destructive nature of the disease.[10] These complications include:
- Tooth loss
- Further loss of attachment
- Extensive soft tissue necrosis
- Alveolar bone exposure
- Sequestration of bone fragments
- Bacteremia
- Weight loss and dehydration[4][22]
Deterrence and Patient Education
Educate at-risk patients on modifying risk factors such as oral hygiene and nutrition to prevent the occurrence and recurrence of necrotizing periodontal diseases. Interdisciplinary healthcare professionals should work together to identify, monitor, treat, and manage patients with necrotizing periodontal diseases and treat underlying conditions. Patients should be aware of the importance of rapid identification of signs and symptoms of NPDs to provide immediate and effective treatment to limit the extent of the destruction.
Enhancing Healthcare Team Outcomes
Necrotizing periodontal diseases are a clinical diagnosis. They are characterized by their aggressive nature and rapid onset, leading to extreme pain, gingival necrosis, interdental ulceration, and in more advanced stages, osteonecrosis [2]. Any general dentist should be capable of diagnosing and managing NPDs and being aware of risk factors, mainly immunocompromised state and psychological stress, tobacco smoking, poor oral hygiene, and malnutrition. General dentists also play an invaluable role by acting as counselors for smoking cessation, proper nutrition, and stress reduction practices.
Although general dentists are responsible for providing the acute and initial management of NPDs, referrals to a periodontal specialist or oral surgeon should be made to manage the disease sequelae. It is imperative that dental and medical professionals simultaneously address the oral complications and underlying systemic conditions. An interprofessional approach will improve NPDs patients' outcomes. [Level 5]